Global Information
Global InformationToluene information
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Toluene[2] | |||
| Systematic IUPAC name
Methylbenzene | |||
| Other names
Methyl benzene[1]
Methylcyclohexa-1,3,5-triene Benzylane Phenylmethane Toluol Anisen | |||
| Identifiers | |||
CAS Number
|
| ||
3D model (JSmol)
|
| ||
| Abbreviations | PhMe MePh BnH Tol | ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| DrugBank |
| ||
| ECHA InfoCard | 100.003.297 | ||
IUPHAR/BPS
|
| ||
| KEGG |
| ||
PubChem CID
|
| ||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 1294 | ||
CompTox Dashboard (EPA)
|
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula
|
C6H5CH3 | ||
| Molar mass | 92.141 g·mol−1 | ||
| Appearance | Colorless liquid[3] | ||
| Odor | sweet, pungent, benzene-like[4] | ||
| Density | 0.8623 g/mL (25 °C)[1] | ||
| Melting point | −95.0 °C (−139.0 °F; 178.2 K)[1] | ||
| Boiling point | 110.60 °C (231.08 °F; 383.75 K)[1] | ||
Solubility in water
|
0.54 g/L (5 °C) 0.519 g/L (25 °C) 0.63 g/L (45 °C) 1.2 g/L (90 °C)[5] | ||
| log P | 2.73[6] | ||
| Vapor pressure | 2.8 kPa (20 °C)[4] | ||
Magnetic susceptibility (χ)
|
−66.1·10−6 cm3/mol[7] | ||
| Thermal conductivity | 0.1310 W/(m·K) (25 °C)[8] | ||
Refractive index (nD)
|
1.4941 (25 °C)[1] | ||
| Viscosity | 0.560 mPa·s (25 °C)[9] | ||
| Structure | |||
Dipole moment
|
0.375 D[10] | ||
| Thermochemistry[11] | |||
Heat capacity (C)
|
157.3 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
12.4 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
3.910 MJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly flammable | ||
| GHS labelling: | |||
Pictograms
|
  
| ||
Signal word
|
Danger | ||
Hazard statements
|
H225, H304, H315, H336, H361d, H373 | ||
Precautionary statements
|
P210, P240, P301+P310, P302+P352, P308+P313, P314, P403+P233 | ||
| NFPA 704 (fire diamond) | 
2
3
0 | ||
| Flash point | 4 °C (39 °F; 277 K)[12] | ||
Autoignition
temperature |
480[12] °C (896 °F; 753 K) | ||
| Explosive limits | 1.1–7.1%[12] | ||
Threshold limit value (TLV)
|
50 mL/m3, 190 mg/m3 | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
>26700 ppm (rat, 1 h) 400 ppm (mouse, 24 h)[13] | ||
LCLo (lowest published)
|
55,000 ppm (rabbit, 40 min)[13] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 200 ppm C 300 ppm 500 ppm (10-minute maximum peak)[4] | ||
REL (Recommended)
|
TWA 100 ppm (375 mg/m3) ST 150 ppm (560 mg/m3)[4] | ||
IDLH (Immediate danger)
|
500 ppm[4] | ||
| Safety data sheet (SDS) | SIRI.org | ||
| Related compounds | |||
Related aromatic hydrocarbons
|
benzene xylene naphthalene | ||
Related compounds
|
methylcyclohexane | ||
| Supplementary data page | |||
| Toluene (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
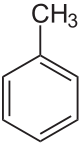
Toluene (/ˈtɒl.juiːn/), also known as toluol (/ˈtɒl.ju.ɒl, -ɔːl, -oʊl/), is a substituted aromatic hydrocarbon[15] with the chemical formula C6H5CH3, often abbreviated as PhCH3, where Ph stands for phenyl group. It is a colorless, water-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.
As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant[16] and has the potential of causing severe neurological harm.[17][18]
- ^ a b c d e Haynes, p. 3.514
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 139. doi:10.1039/9781849733069-00130. ISBN 978-0-85404-182-4.
Toluene and xylene are preferred IUPAC names, but are not freely substitutable; toluene is substitutable under certain conditions, but only for general nomenclature (see P-15.1.8 for a general substitution rules for retained names).
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0619". National Institute for Occupational Safety and Health (NIOSH).
- ^ Haynes, p. 5.164
- ^ Haynes, p. 5.176
- ^ Haynes, p. 3.579
- ^ Haynes, p. 6.258
- ^ Haynes, p. 6.246
- ^ Haynes, p. 9.66
- ^ Haynes, pp. 5.39, 5.67
- ^ a b c Haynes, p. 16.30
- ^ a b "Toluene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "NFPA Chemicals". New Environment, Inc. Archived from the original on November 14, 2021. Retrieved March 13, 2015.
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page). - ^ McKeown NJ (February 1, 2015). Tarabar A (ed.). "Toluene Toxicity, Background, Pathophysiology, Epidemiology". WebMD Health Professional Network. Archived from the original on March 9, 2016. Retrieved March 22, 2016.
- ^ Streicher HZ, Gabow PA, Moss AH, Kono D, Kaehny WD (June 1981). "Syndromes of toluene sniffing in adults". Annals of Internal Medicine. 94 (6): 758–62. doi:10.7326/0003-4819-94-6-758. PMID 7235417.
- ^ Devathasan G, Low D, Teoh PC, Wan SH, Wong PK (February 1984). "Complications of chronic glue (toluene) abuse in adolescents". Australian and New Zealand Journal of Medicine. 14 (1): 39–43. doi:10.1111/j.1445-5994.1984.tb03583.x. PMID 6087782.