 Global Information
Global InformationDapagliflozin information
 | |||
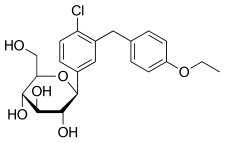 Haworth projection (bottom) | |||
| |||
| Clinical data | |||
|---|---|---|---|
| Pronunciation | /ˌdæpəɡlɪˈfloʊzɪn/ DAP-ə-glif-LOH-zin | ||
| Trade names | Forxiga, Farxiga, others | ||
| Other names | BMS-512148; (1S)-1,5-anhydro-1-C-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-D-glucitol | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a614015 | ||
| License data |
| ||
| Pregnancy category |
| ||
| Routes of administration | By mouth | ||
| Drug class | Sodium-glucose co-transporter 2 (SGLT2) inhibitor | ||
| ATC code |
| ||
| Legal status | |||
| Legal status |
| ||
| Pharmacokinetic data | |||
| Bioavailability | 78% (after 10 mg dose) | ||
| Protein binding | ~91% | ||
| Metabolism | UGT1A9 (major), CYP (minor) | ||
| Metabolites | Dapagliflozin 3-O-glucuronide (inactive) | ||
| Elimination half-life | ~12.9 hours | ||
| Excretion | Urine (75%), feces (21%)[6] | ||
| Identifiers | |||
IUPAC name
| |||
| CAS Number |
| ||
| PubChem CID |
| ||
| IUPHAR/BPS |
| ||
| DrugBank |
| ||
| ChemSpider |
| ||
| UNII |
| ||
| KEGG |
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| CompTox Dashboard (EPA) |
| ||
| ECHA InfoCard | 100.167.331 | ||
| Chemical and physical data | |||
| Formula | C21H25ClO6 | ||
| Molar mass | 408.88 g·mol−1 | ||
| 3D model (JSmol) |
| ||
SMILES
| |||
InChI
| |||
Dapagliflozin, sold under the brand names Farxiga (US) and Forxiga (EU) among others, is a medication used to treat type 2 diabetes.[6][7][9] It is also used to treat adults with heart failure and chronic kidney disease.[10][11][7] It reversibly inhibits sodium-glucose co-transporter 2 (SGLT-2) in the renal proximal convoluted tubule to reduce glucose reabsorption and increase urinary glucose excretion.[12]
Common side effects include hypoglycaemia (low blood sugar), urinary tract infections, genital infections, and volume depletion (reduced amount of water in the body).[13] Diabetic ketoacidosis is a common side effect in people with type 1 diabetes.[14] Serious but rare side effects include Fournier gangrene.[15]
It was developed by Bristol-Myers Squibb in partnership with AstraZeneca. It is on the World Health Organization's List of Essential Medicines.[16] In 2021, it was the 187th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[17][18] Dapagliflozin is available as a generic medication.
- ^ "Dapagliflozin (Farxiga) Use During Pregnancy". Drugs.com. 30 August 2018. Archived from the original on 17 April 2021. Retrieved 5 May 2020.
- ^ "Forxiga dapagliflozin (as propanediol monohydrate) 10 mg film-coated tablet blister pack (180147)". Therapeutic Goods Administration (TGA). 27 May 2022. Retrieved 20 April 2024.
- ^ "AusPAR: Dapagliflozin". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 20 April 2024.
- ^ "AusPAR: Dapagliflozin (as propanediol monohydrate)". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 20 April 2024.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Retrieved 1 April 2024.
- ^ a b c "Farxiga- dapagliflozin tablet, film coated". DailyMed. National Institutes of Health, National Library of Medicine, U.S. Department of Health & Human Services. 3 February 2020. Archived from the original on 30 October 2020. Retrieved 5 May 2020.
- ^ a b c Cite error: The named reference
Forxiga EPARwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Dapagliflozin Viatris EPARwas invoked but never defined (see the help page). - ^ "Forxiga (dapagliflozin) 5mg should no longer be used for the treatment of Type 1 Diabetes Mellitus". European Medicines Agency (EMA). 11 November 2021. Archived from the original on 11 November 2021. Retrieved 11 November 2021.
- ^ "FDA approves new treatment for a type of heart failure". U.S. Food and Drug Administration (FDA) (Press release). 5 May 2020. Archived from the original on 6 May 2020. Retrieved 5 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ National Institute for Health and Care Excellence (24 February 2021). "Dapagliflozin for treating chronic heart failure with reduced ejection fraction". NICE Technology Appraisal Auidance [TA679]. NICE. Archived from the original on 9 May 2021. Retrieved 9 May 2021.
- ^ "BNF: Dapagliflozin". NICE. Retrieved 2 February 2024.
- ^ Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF (October 2014). "Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events". Drug Safety. 37 (10): 815–829. doi:10.1007/s40264-014-0213-4. PMID 25096959. S2CID 24064402.
- ^ Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, Thorén F, et al. (DEPICT-1 Investigators) (December 2018). "Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study". Diabetes Care. 41 (12): 2552–2559. doi:10.2337/dc18-1087. PMID 30352894. S2CID 53027785.
- ^ Hu Y, Bai Z, Tang Y, Liu R, Zhao B, Gong J, et al. (2020). "Fournier Gangrene Associated with Sodium-Glucose Cotransporter-2 Inhibitors: A Pharmacovigilance Study with Data from the U.S. FDA Adverse Event Reporting System". Journal of Diabetes Research. 2020: 3695101. doi:10.1155/2020/3695101. PMC 7368210. PMID 32695827.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Dapagliflozin - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
