 Global Information
Global InformationGlucose information
 Skeletal formula of d-glucose
| |
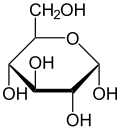 Haworth projection of α-d-glucopyranose
| |
 Fischer projection of d-glucose
| |
| Names | |
|---|---|
| Pronunciation | /ˈɡluːkoʊz/, /ɡluːkoʊs/ |
| IUPAC name
Allowed trivial names:[1]
| |
| Preferred IUPAC name
PINs are not identified for natural products. | |
Systematic IUPAC name
| |
| Other names
Blood sugars
Dextrose Corn sugar d-Glucose Grape sugar | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| 3DMet |
|
| Abbreviations | Glc |
Beilstein Reference
|
1281604 |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| EC Number |
|
Gmelin Reference
|
83256 |
IUPHAR/BPS
|
|
| KEGG |
|
| MeSH | Glucose |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C6H12O6 |
| Molar mass | 180.156 g/mol |
| Appearance | White powder |
| Density | 1.54 g/cm3 |
| Melting point | α-d-Glucose: 146 °C (295 °F; 419 K) β-d-Glucose: 150 °C (302 °F; 423 K) |
Solubility in water
|
909 g/L (25 °C (77 °F)) |
Magnetic susceptibility (χ)
|
−101.5×10−6 cm3/mol |
Dipole moment
|
8.6827 |
| Thermochemistry | |
Heat capacity (C)
|
218.6 J/(K·mol)[2] |
Std molar
entropy (S⦵298) |
209.2 J/(K·mol)[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−1271 kJ/mol[3] |
Heat of combustion, higher value (HHV)
|
2,805 kJ/mol (670 kcal/mol) |
| Pharmacology | |
ATC code
|
B05CX01 (WHO) V04CA02 (WHO), V06DC01 (WHO) |
| Hazards | |
| NFPA 704 (fire diamond) | 
0
1
0 |
| Safety data sheet (SDS) | ICSC 08655 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Glucose is a sugar with the molecular formula C6H12O6. Glucose is overall the most abundant monosaccharide,[4] a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. Glucose is used by plants to make cellulose—the most abundant carbohydrate in the world—for use in cell walls, and by all living organisms to make ATP(Adenosine Triphosphate), which is used by the cell as energy.[5][6][7]
In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar.[5][7] The naturally occurring form of glucose is d-glucose, while its stereoisomer l-glucose is produced synthetically in comparatively small amounts and is less biologically active.[7] Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. In animals, glucose is released from the breakdown of glycogen in a process known as glycogenolysis.
Glucose, as intravenous sugar solution, is on the World Health Organization's List of Essential Medicines.[8] It is also on the list in combination with sodium chloride (table salt).[8]
The name glucose is derived from Ancient Greek γλεῦκος (gleûkos) 'wine, must', from γλυκύς (glykýs) 'sweet'.[9][10] The suffix -ose is a chemical classifier denoting a sugar.
- ^ Nomenclature of Carbohydrates (Recommendations 1996) | 2-Carb-2 Archived 2023-08-27 at the Wayback Machine. iupac.qmul.ac.uk.
- ^ a b Boerio-Goates J (1991), "Heat-capacity measurements and thermodynamic functions of crystalline α-D-glucose at temperatures from 10K to 340K", J. Chem. Thermodyn., 23 (5): 403–09, doi:10.1016/S0021-9614(05)80128-4
- ^ Ponomarev VV, Migarskaya LB (1960), "Heats of combustion of some amino-acids", Russ. J. Phys. Chem. (Engl. Transl.), 34: 1182–83
- ^ Domb AJ, Kost J, Wiseman D (1998-02-04). Handbook of Biodegradable Polymers. CRC Press. p. 275. ISBN 978-1-4200-4936-7.
- ^ a b "NCATS Inxight Drugs — DEXTROSE, UNSPECIFIED FORM". Archived from the original on 2023-12-11. Retrieved 2024-03-18.
- ^ Kamide K (2005). Cellulose products and Cellulose Derivatives: Molecular Characterization and its Applications (1st ed.). Amsterdam: Elsevier. p. 1. ISBN 978-0-08-045444-3. Retrieved 13 May 2021.
- ^ a b c "L-glucose". Biology Articles, Tutorials & Dictionary Online. 2019-10-07. Archived from the original on 2022-05-25. Retrieved 2022-05-06.
- ^ a b World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Online Etymology Dictionary". Etymonline.com. Archived from the original on 2016-11-26. Retrieved 2016-11-25.
- ^ Thénard, Gay-Lussac, Biot, and Dumas (1838) "Rapport sur un mémoire de M. Péligiot, intitulé: Recherches sur la nature et les propriétés chimiques des sucres". Archived 2015-12-06 at the Wayback Machine (Report on a memoir of Mr. Péligiot, titled: Investigations on the nature and chemical properties of sugars), Comptes rendus, 7 : 106–113. From page 109. Archived 2015-12-06 at the Wayback Machine: "Il résulte des comparaisons faites par M. Péligot, que le sucre de raisin, celui d'amidon, celui de diabètes et celui de miel ont parfaitement la même composition et les mêmes propriétés, et constituent un seul corps que nous proposons d'appeler Glucose (1). ... (1) γλευχος, moût, vin doux." It follows from the comparisons made by Mr. Péligot, that the sugar from grapes, that from starch, that from diabetes and that from honey have exactly the same composition and the same properties, and constitute a single substance that we propose to call glucose (1) ... (1) γλευχος, must, sweet wine.