Global Information
Global InformationAcetaldehyde information
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Acetaldehyde[3] | |||
| Systematic IUPAC name
Ethanal[3] | |||
| Other names
Acetic aldehyde
Ethyl aldehyde[1] Acetylaldehyde[2] | |||
| Identifiers | |||
CAS Number
|
| ||
3D model (JSmol)
|
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.000.761 | ||
| EC Number |
| ||
IUPHAR/BPS
|
| ||
| KEGG |
| ||
PubChem CID
|
| ||
| RTECS number |
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
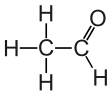
Chemical formula
|
C2H4O | ||
| Molar mass | 44.053 g·mol−1 | ||
| Appearance | Colourless gas or liquid | ||
| Odor | Ethereal | ||
| Density | 0.784 g·cm−3 (20 °C)[4]
0.7904–0.7928 g·cm−3 (10 °C)[4] | ||
| Melting point | −123.37 °C (−190.07 °F; 149.78 K) | ||
| Boiling point | 20.2 °C (68.4 °F; 293.3 K) | ||
Solubility in water
|
miscible | ||
| Solubility | miscible with ethanol, ether, benzene, toluene, xylene, turpentine, acetone slightly soluble in chloroform | ||
| log P | -0.34 | ||
| Vapor pressure | 740 mmHg (20 °C)[5] | ||
| Acidity (pKa) | 13.57 (25 °C, H2O)[6] | ||
Magnetic susceptibility (χ)
|
-.5153−6 cm3/g | ||
Refractive index (nD)
|
1.3316 | ||
| Viscosity | 0.21 mPa-s at 20 °C (0.253 mPa-s at 9.5 °C)[7] | ||
| Structure | |||
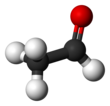
Molecular shape
|
trigonal planar (sp2) at C1 tetrahedral (sp3) at C2 | ||
Dipole moment
|
2.7 D | ||
| Thermochemistry[8] | |||
Heat capacity (C)
|
89 J·mol−1·K−1 | ||
Std molar
entropy (S⦵298) |
160.2 J·mol−1·K−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−192.2 kJ·mol−1 | ||
Gibbs free energy (ΔfG⦵)
|
-127.6 kJ·mol−1 | ||
| Related compounds | |||
Related aldehydes
|
Formaldehyde Propionaldehyde | ||
Related compounds
|
Ethylene oxide | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
potential occupational carcinogen[10] | ||
| GHS labelling: | |||
Pictograms
|
   [9] [9]
| ||
Hazard statements
|
H224, H319, H335, H351[9] | ||
Precautionary statements
|
P210, P261, P281, P305+P351+P338[9] | ||
| NFPA 704 (fire diamond) | 
3
4
3 | ||
| Flash point | −39.00 °C; −38.20 °F; 234.15 K | ||
Autoignition
temperature |
175.00 °C; 347.00 °F; 448.15 K[5] | ||
| Explosive limits | 4.0–60% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1930 mg/kg (rat, oral) | ||
LC50 (median concentration)
|
13,000 ppm (rat), 17,000 ppm (hamster), 20,000 ppm (rat)[10] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
200 ppm (360 mg/m3)[5] | ||
IDLH (Immediate danger)
|
2000 ppm[5][10] | ||
| Safety data sheet (SDS) | HMDB | ||
| Supplementary data page | |||
| Acetaldehyde (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated as MeCHO. It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit,[11] and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke.[12] Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen.[13] Acetaldehyde is "one of the most frequently found air toxins with cancer risk greater than one in a million".[14]
- ^ SciFinderScholar (accessed 4 November 2009). Acetaldehyde (75-07-0) Substance Detail.
- ^ Molecular Pathology and Diagnostics of Cancer p. 190
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 908. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ a b Stoffdaten Acetaldehyd bei Celanese Chemicals. Archived 17 May 2008 at the Wayback Machine as of December 1999.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0001". National Institute for Occupational Safety and Health (NIOSH).
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–88. ISBN 9781498754293.
- ^ "Acetaldehyde".
- ^ John Rumble (18 June 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 5–3. ISBN 978-1138561632.
- ^ a b c Sigma-Aldrich Co., Acetaldehyde. Retrieved on 2022-02-15.
- ^ a b c "Acetaldehyde". NIOSH. 4 December 2014. Retrieved 12 February 2015.
- ^ Uebelacker, Michael; Lachenmeier, Dirk (13 June 2011). "Quantitative Determination of Acetaldehyde in Foods Using Automated Digestion with Simulated Gastric Fluid Followed by Headspace Gas Chromatography". Journal of Automated Methods and Management in Chemistry. 2011: 907317. doi:10.1155/2011/907317. PMC 3124883. PMID 21747735.
- ^ "Chemicals in the Environment: Acetaldehyde (CAS NO. 75-07-0)". epa.gov. Office of Pollution Prevention and Toxics, United States Environmental Protection Agency. August 1994. Archived from the original on 17 August 2002. Retrieved 22 January 2011.
- ^ List of IARC Group 1 carcinogens
- ^ Zhou, Ying; Li, Chaoyang; Huijbregts, Mark A. J.; Mumtaz, M. Moiz (7 October 2015). "Carcinogenic Air Toxics Exposure and Their Cancer-Related Health Impacts in the United States". PLOS ONE. 10 (10): e0140013. Bibcode:2015PLoSO..1040013Z. doi:10.1371/journal.pone.0140013. PMC 4596837. PMID 26444872.