 Global Information
Global InformationPerfluorooctanoic acid information
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Pentadecafluorooctanoic acid | |
| Other names
Perfluorooctanoic acid, PFOA, C8, Perfluorooctanoate, PFO, Perfluorocaprylic acid, C8-PFCA, FC-143, F-n-octanoic acid
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.005.817 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
InChI
| |
SMILES
| |
| Properties | |
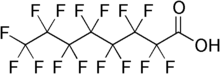
Chemical formula
|
C8HF15O2 |
| Molar mass | 414.07 g/mol |
| Appearance | White solid |
| Density | 1.8 g/cm3[1] |
| Melting point | 40 to 50 °C (104 to 122 °F; 313 to 323 K)[1] |
| Boiling point | 189 to 192 °C (372 to 378 °F; 462 to 465 K)[1] |
Solubility in water
|
Soluble, 9.5 g/L (PFO)[2] |
| Solubility in other solvents | Polar organic solvents |
| Acidity (pKa) | ~0[3][4][5] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Strong acid, known carcinogen, persistent organic pollutant |
| GHS labelling: | |
Pictograms
|
  
|
Signal word
|
Danger |
Hazard statements
|
H302, H318, H332, H351, H360, H362, H372 |
Precautionary statements
|
P201, P202, P260, P261, P263, P264, P270, P271, P280, P281, P301+P312, P304+P312, P304+P340, P305+P351+P338, P308+P313, P310, P312, P314, P330, P405, P501 |
| NFPA 704 (fire diamond) | 
3
0
0 |
| Safety data sheet (SDS) | [1] |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Perfluorooctanoic acid (PFOA; conjugate base perfluorooctanoate; also known colloquially as C8, for its 8-carbon chain structure) is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes and as a material feedstock. PFOA is considered a surfactant, or fluorosurfactant, due to its chemical structure, which consists of a perfluorinated, n-heptyl "tail group" and a carboxylic acid "head group". The head group can be described as hydrophilic while the fluorocarbon tail is both hydrophobic and lipophobic.[6]
The International Agency for Research on Cancer (IARC) has classified PFOA as carcinogenic to humans.[7] PFOA is one of many synthetic organofluorine compounds collectively known as per- and polyfluoroalkyl substances (PFASs). Many PFAS such as PFOS, PFOA are a concern because they do not break down via natural processes and are commonly described as persistent organic pollutants or "forever chemicals".[8] They can also move through soils and contaminate drinking water sources and can build up (bioaccumulate) in fish and wildlife.[8] Residues have been detected in humans and wildlife.[9][10]
PFOA is used in several industrial applications, including carpeting, upholstery, apparel, floor wax, textiles, fire fighting foam and sealants. PFOA serves as a surfactant in the emulsion polymerization of fluoropolymers and as a building block for the synthesis of perfluoroalkyl-substituted compounds, polymers, and polymeric materials. PFOA has been manufactured since the 1940s in industrial quantities.[11] It is also formed by the degradation of precursors such as some fluorotelomers. PFOA is used as a surfactant because it can lower the surface tension of water more than hydrocarbon surfactants while having exceptional stability due to having perfluoroalkyl tail group.[6][12] The stability of PFOA is desired industrially but is a cause of concern environmentally.
The primary manufacturer of perfluorooctanesulfonic acid (PFOS), the 3M Company (known as Minnesota Mining and Manufacturing Company from 1902 to 2002), began a production phase-out in 2002 in response to concerns expressed by the United States Environmental Protection Agency (EPA).[13]: 2 Eight other companies agreed to gradually phase out the manufacturing of the chemical by 2015.[13]: 3
By 2014, EPA had listed PFOA and perfluorooctanesulfonates (salts of perfluorooctanesulfonic acid, PFOS) as emergent contaminants:
PFOA and PFOS are extremely persistent in the environment and resistant to typical environmental degradation processes. [They] are widely distributed across the higher trophic levels and are found in soil, air and groundwater at sites across the United States. The toxicity, mobility and bioaccumulation potential of PFOS and PFOA pose potential adverse effects for the environment and human health.[13]: 1
- ^ a b c d Record of Perfluorooctanoic acid in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 5 November 2008.
- ^ Cite error: The named reference
Prevedouros2006was invoked but never defined (see the help page). - ^ Goss K. U. (July 2008). "The pKa values of PFOA and other highly fluorinated carboxylic acids". Environ. Sci. Technol. 42 (2): 456–458. Bibcode:2008EnST...42..456G. doi:10.1021/es702192c. PMID 18284146.
- ^ Cheng J, Psillakis E, Hoffmann MR, Colussi AJ (July 2009). "Acid dissociation versus molecular association of perfluoroalkyl oxoacids: Environmental implications". J. Phys. Chem. A. 113 (29): 8152–8156. Bibcode:2009JPCA..113.8152C. doi:10.1021/jp9051352. PMID 19569653.
- ^ Rayne S, Forest K (June 2010). "Theoretical studies on the pKa values of perfluoroalkyl carboxylic acids". J. Mol. Struct. (Theochem). 949 (1–3): 60–69. doi:10.1016/j.theochem.2010.03.003.
- ^ a b Lemal DM (January 2004). "Perspective on fluorocarbon chemistry". J. Org. Chem. 69 (1): 1–11. doi:10.1021/jo0302556. PMID 14703372.
- ^ Zahm S, Bonde JP, Chiu WA, Hoppin J, Kanno J, Abdallah M, et al. (November 2023). "Carcinogenicity of perfluorooctanoic acid and perfluorooctanesulfonic acid". The Lancet. Oncology. 25 (1): 16–17. doi:10.1016/S1470-2045(23)00622-8. PMID 38043561. S2CID 265571186.
- ^ a b "Per- and Polyfluorinated Substances (PFAS) Factsheet | National Biomonitoring Program | CDC". www.cdc.gov. 2022-05-03. Retrieved 2024-04-12.
- ^ "Per- and Polyfluorinated Substances (PFAS) Factsheet | National Biomonitoring Program | CDC". www.cdc.gov. 2022-05-03. Retrieved 2024-04-12.
- ^ "Emerging chemical risks in Europe — 'PFAS' — European Environment Agency". www.eea.europa.eu. Retrieved 2024-04-12.
- ^ Lindstrom AB, Strynar MJ, Libelo EL (2011-08-25). "Polyfluorinated Compounds: Past, Present, and Future". Environ. Sci. Technol. 45 (19): 7954–7961. Bibcode:2011EnST...45.7954L. doi:10.1021/es2011622. PMID 21866930.
- ^ Cite error: The named reference
Salager2002was invoked but never defined (see the help page). - ^ a b c Emerging Contaminants Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) (Report). EPA. March 2014. 505-F-14-001. Fact sheet.