 Global Information
Global InformationCefiderocol information
 | |
| Clinical data | |
|---|---|
| Trade names | Fetroja, Fetcroja |
| Other names | RSC-649266 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620008 |
| License data |
|
| Routes of administration | Intravenous infusion |
| Drug class | Siderophore cephalosporins |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 56–58%[4] |
| Elimination half-life | 2.8 hours |
| Excretion | mainly kidney (60–70% unchanged) |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
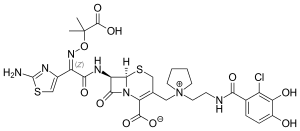
| Formula | C30H34ClN7O10S2 |
| Molar mass | 752.21 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
Cefiderocol, sold under the brand name Fetroja among others, is an antibiotic used to treat complicated urinary tract infections when no other options are available.[5] It is indicated for the treatment of multi-drug-resistant Gram-negative bacteria including Pseudomonas aeruginosa.[6][7][8] It is given by injection into a vein.[1]
Common side effects include diarrhea, infusion site reactions, constipation and rash.[9]
Cefiderocol is in the cephalosporin family of medications.[5][10] It was approved for medical use in the United States in November 2019, and in the European Union in April 2020.[5][11][2] In September 2020, cefiderocol (Fetroja) received FDA approval[12] as supplemental New Drug Application (sNDA) for treatment of hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) when caused by Gram-negative bacteria resistant to other antibiotics. It is on the World Health Organization's List of Essential Medicines.[13]
- ^ a b "Fetroja- cefiderocol sulfate tosylate injection, powder, for solution". DailyMed. 19 November 2019. Retrieved 29 April 2020.
- ^ a b Cite error: The named reference
Fetcroja EPARwas invoked but never defined (see the help page). - ^ "Fetcroja Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C (May 2017). "Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects With Renal Impairment". Journal of Clinical Pharmacology. 57 (5): 584–591. doi:10.1002/jcph.841. PMC 5412848. PMID 27874971.
- ^ a b c Cite error: The named reference
FDA2019was invoked but never defined (see the help page). - ^ Choi JJ, McCarthy MW (February 2018). "Cefiderocol: a novel siderophore cephalosporin". Expert Opinion on Investigational Drugs. 27 (2): 193–197. doi:10.1080/13543784.2018.1426745. PMID 29318906. S2CID 205768562.
- ^ Aoki T, Yoshizawa H, Yamawaki K, Yokoo K, Sato J, Hisakawa S, et al. (July 2018). "Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship". European Journal of Medicinal Chemistry. 155: 847–868. doi:10.1016/j.ejmech.2018.06.014. PMID 29960205. S2CID 49682995.
- ^ Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JC, Ariyasu M, et al. (December 2018). "Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial". The Lancet. Infectious Diseases. 18 (12): 1319–1328. doi:10.1016/S1473-3099(18)30554-1. PMID 30509675. S2CID 54552812.
- ^ "Drug Trials Snapshot: Fetroja". U.S. Food and Drug Administration (FDA). 14 November 2019. Retrieved 29 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, et al. (February 2019). "Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli". Drugs. 79 (3): 271–289. doi:10.1007/s40265-019-1055-2. PMID 30712199. S2CID 59541210.
- ^ "Drug Approval Package: Fetroja (cefiderocol)". U.S. Food and Drug Administration (FDA). 19 December 2019. Retrieved 29 April 2020.
- ^ "FDA Approves Fetroja (cefiderocol) for the Treatment of Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia". Drugs.com. Retrieved 29 September 2020.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.