Global Information
Global InformationRibose information
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
D-Ribose
| |||
| Systematic IUPAC name
(2R,3R,4S,5R)-5-(hydroxymethyl)oxolane-2,3,4-triol | |||
| Other names
d-Ribose
| |||
| Identifiers | |||
CAS Number
|
| ||
3D model (JSmol)
|
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| DrugBank |
| ||
| EC Number |
| ||
PubChem CID
|
| ||
| UNII |
| ||
InChI
| |||
SMILES
| |||
| Properties[1][2] | |||
Chemical formula
|
C5H10O5 | ||
| Molar mass | 150.13 | ||
| Appearance | White solid | ||
| Melting point | 95 °C (203 °F; 368 K) | ||
Solubility in water
|
100 g/L (25 °C, 77 °F) | ||
Chiral rotation ([α]D)
|
−21.5° (H2O) | ||
| Related compounds | |||
Related aldopentoses
|
Arabinose Xylose Lyxose | ||
Related compounds
|
Deoxyribose | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||

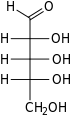
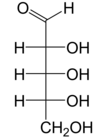
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, d-ribose, is a component of the ribonucleotides from which RNA is built, and so this compound is necessary for coding, decoding, regulation and expression of genes. It has a structural analog, deoxyribose, which is a similarly essential component of DNA. l-ribose is an unnatural sugar that was first prepared by Emil Fischer and Oscar Piloty in 1891.[3] It was not until 1909 that Phoebus Levene and Walter Jacobs recognised that d-ribose was a natural product, the enantiomer of Fischer and Piloty's product, and an essential component of nucleic acids.[4][5][6] Fischer chose the name "ribose" as it is a partial rearrangement of the name of another sugar, arabinose, of which ribose is an epimer at the 2' carbon; both names also relate to gum arabic, from which arabinose was first isolated and from which they prepared l-ribose.[6][7]
Right: Fischer projection of the open chain forms of d- and l- ribose
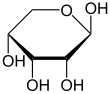
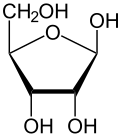
Like most sugars, ribose exists as a mixture of cyclic forms in equilibrium with its linear form, and these readily interconvert especially in aqueous solution.[8] The name "ribose" is used in biochemistry and biology to refer to all of these forms, though more specific names for each are used when required. In its linear form, ribose can be recognised as the pentose sugar with all of its hydroxyl functional groups on the same side in its Fischer projection. d-Ribose has these hydroxyl groups on the right hand side and is associated with the systematic name (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal,[9] whilst l-ribose has its hydroxyl groups appear on the left hand side in a Fischer projection. Cyclisation of ribose occurs via hemiacetal formation due to attack on the aldehyde by the C4' hydroxyl group to produce a furanose form or by the C5' hydroxyl group to produce a pyranose form. In each case, there are two possible geometric outcomes, named as α- and β- and known as anomers, depending on the stereochemistry at the hemiacetal carbon atom (the "anomeric carbon"). At room temperature, about 76% of d-ribose is present in pyranose forms[8]: 228 (α:β = 1:2)[10] and 24% in the furanose forms[8]: 228 (α:β = 1:3),[10] with only about 0.1% of the linear form present.[11][12]
The ribonucleosides adenosine, cytidine, guanosine, and uridine are all derivatives of β-d-ribofuranose. Metabolically-important species that include phosphorylated ribose include ADP, ATP, coenzyme A,[8]: 228–229 and NADH. cAMP and cGMP serve as secondary messengers in some signaling pathways and are also ribose derivatives. The ribose moiety appears in some pharmaceutical agents, including the antibiotics neomycin and paromomycin.[10]
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 8205
- ^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-506. ISBN 0-8493-0462-8.
- ^ Fischer, Emil; Piloty, Oscar (1891). "Ueber eine neue Pentonsäure und die zweite inactive Trioxyglutarsäure" [About a new pentonic acid and the second inactive trioxyglutaric acid]. Berichte der deutschen chemischen Gesellschaft (in German). 24 (2): 4214–4225. doi:10.1002/cber.189102402322. Archived from the original on 4 June 2020. Retrieved 12 March 2020.
- ^ Levene, P. A.; Jacobs, W. A. (1909). "Über Inosinsäure" [About inosic acid]. Berichte der deutschen chemischen Gesellschaft (in German). 42 (1): 1198–1203. doi:10.1002/cber.190904201196.
- ^ Levene, P. A.; Jacobs, W. A. (1909). "Über die Pentose in den Nucleinsäuren" [About the pentose in the nucleic acids]. Berichte der deutschen chemischen Gesellschaft (in German). 42 (3): 3247–3251. doi:10.1002/cber.19090420351.
- ^ a b Jeanloz, Roger W.; Fletcher, Hewitt G. (1951). "The Chemistry of Ribose". In Hudson, Claude S.; Cantor, Sidney M. (eds.). Advances in Carbohydrate Chemistry. Vol. 6. Academic Press. pp. 135–174. doi:10.1016/S0096-5332(08)60066-1. ISBN 9780080562650. PMID 14894350. Archived from the original on 26 October 2023. Retrieved 15 December 2019.
- ^ Nechamkin, Howard (1958). "Some interesting etymological derivations of chemical terminology". Science Education. 42 (5): 463–474. Bibcode:1958SciEd..42..463N. doi:10.1002/sce.3730420523.
- ^ a b c d Dewick, Paul M. (2013). "Oxygen as a Nucleophile: Hemicetals, Hemiketals, Acetals and Ketals". Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal Chemistry and Biological Chemistry. John Wiley & Sons. pp. 224–234. ISBN 9781118681961. Archived from the original on 26 October 2023. Retrieved 15 December 2019.
- ^ Leigh, Jeffery (July–August 2012). "Non-IUPAC Nomenclature Systems". Chemistry International. 34 (4). International Union of Pure and Applied Chemistry. Archived from the original on 5 December 2019. Retrieved 15 December 2019.
- ^ a b c Bhutani, S. P. (2019). "Aldopentoses—The Sugars of Nucleic Acids". Chemistry of Biomolecules (2nd ed.). CRC Press. pp. 63–65. ISBN 9781000650907. Archived from the original on 26 October 2023. Retrieved 15 December 2019.
- ^ Cite error: The named reference
DrewEtAlwas invoked but never defined (see the help page). - ^ de Wulf, P.; Vandamme, E. J. (1997). "Microbial Synthesis of ᴅ-Ribose: Metabolic Deregulation and Fermentation Process". Advances in Applied Microbiology. 44: 167–214. doi:10.1016/S0065-2164(08)70462-3. ISBN 9780120026449.