 Global Information
Global InformationGlyphosate information
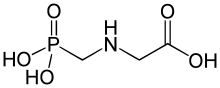 Idealised skeletal formula
| |
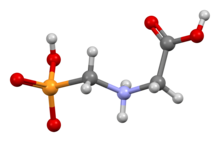 Ball-and-stick model of the zwitterion based on the crystal structure[1][2]
| |
| Names | |
|---|---|
| Pronunciation | /ˈɡlɪfəseɪt, ˈɡlaɪfə-/,[3] /ɡlaɪˈfɒseɪt/[4][5] |
| IUPAC name
N-(Phosphonomethyl)glycine
| |
| Systematic IUPAC name
[(Phosphonomethyl)amino]acetic acid | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
Beilstein Reference
|
2045054 |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.012.726 |
| EC Number |
|
Gmelin Reference
|
279222 |
| KEGG |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 3077 2783 |
CompTox Dashboard (EPA)
|
|
InChI
| |
SMILES
| |
| Properties[6] | |
Chemical formula
|
C3H8NO5P |
| Molar mass | 169.073 g·mol−1 |
| Appearance | white crystalline powder |
| Density | 1.704 (20 °C) |
| Melting point | 184.5 °C (364.1 °F; 457.6 K) |
| Boiling point | 187 °C (369 °F; 460 K) decomposes |
Solubility in water
|
1.01 g/100 mL (20 °C) |
| log P | −2.8 |
| Acidity (pKa) | <2, 2.6, 5.6, 10.6 |
| Hazards[6][7] | |
| GHS labelling: | |
Pictograms
|
 
|
Signal word
|
Danger |
Hazard statements
|
H318, H411 |
Precautionary statements
|
P273, P280, P305+P351+P338, P310, P501 |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | InChem MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Glyphosate (IUPAC name: N-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide and crop desiccant. It is an organophosphorus compound, specifically a phosphonate, which acts by inhibiting the plant enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSP). It is used to kill weeds, especially annual broadleaf weeds and grasses that compete with crops. Its herbicidal effectiveness was discovered by Monsanto chemist John E. Franz in 1970. Monsanto brought it to market for agricultural use in 1974 under the trade name Roundup. Monsanto's last commercially relevant United States patent expired in 2000.
Farmers quickly adopted glyphosate for agricultural weed control, especially after Monsanto introduced glyphosate-resistant Roundup Ready crops, enabling farmers to kill weeds without killing their crops. In 2007, glyphosate was the most used herbicide in the United States' agricultural sector and the second-most used (after 2,4-D) in home and garden, government and industry, and commercial applications.[8] From the late 1970s to 2016, there was a 100-fold increase in the frequency and volume of application of glyphosate-based herbicides (GBHs) worldwide, with further increases expected in the future.
Glyphosate is absorbed through foliage, and minimally through roots, and from there translocated to growing points. It inhibits EPSP synthase, a plant enzyme involved in the synthesis of three aromatic amino acids: tyrosine, tryptophan, and phenylalanine. It is therefore effective only on actively growing plants and is not effective as a pre-emergence herbicide. Crops have been genetically engineered to be tolerant of glyphosate (e.g. Roundup Ready soybean, the first Roundup Ready crop, also created by Monsanto), which allows farmers to use glyphosate as a post-emergence herbicide against weeds.
While glyphosate and formulations such as Roundup have been approved by regulatory bodies worldwide, concerns about their effects on humans and the environment have persisted.[9][10] A number of regulatory and scholarly reviews have evaluated the relative toxicity of glyphosate as an herbicide. The WHO and FAO Joint committee on pesticide residues issued a report in 2016 stating the use of glyphosate formulations does not necessarily constitute a health risk, and giving an acceptable daily intake limit of 1 milligram per kilogram of body weight per day for chronic toxicity.[11]
The consensus among national pesticide regulatory agencies and scientific organizations is that labeled uses of glyphosate have demonstrated no evidence of human carcinogenicity.[12] In March 2015, the World Health Organization's International Agency for Research on Cancer (IARC) classified glyphosate as "probably carcinogenic in humans" (category 2A) based on epidemiological studies, animal studies, and in vitro studies.[10][13][14][15] In contrast, the European Food Safety Authority concluded in November 2015 that "the substance is unlikely to be genotoxic (i.e. damaging to DNA) or to pose a carcinogenic threat to humans", later clarifying that while carcinogenic glyphosate-containing formulations may exist, studies "that look solely at the active substance glyphosate do not show this effect."[16][17] In 2017, the European Chemicals Agency (ECHA) classified glyphosate as causing serious eye damage and as toxic to aquatic life, but did not find evidence implicating it as a carcinogen, a mutagen, toxic to reproduction, nor toxic to specific organs.[18]
- ^ Wilson, C. J. G.; Wood, P. A.; Parsons, S. (2022). "CSD Entry: PHOGLY05". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. doi:10.5517/ccdc.csd.cc2dmhvd. Retrieved November 4, 2023.
- ^ Wilson, Cameron J. G.; Wood, Peter A.; Parsons, Simon (2023). "Discerning subtle high-pressure phase transitions in glyphosate". CrystEngComm. 25 (6): 988–997. doi:10.1039/D2CE01616H. hdl:20.500.11820/e81bbc4f-a6d1-4e16-a288-6eb9ff626485.
- ^ "glyphosate". Merriam-Webster.com Dictionary. Retrieved June 28, 2020.
- ^ "glyphosate". Dictionary.com Unabridged (Online). n.d. Retrieved June 28, 2020.
- ^ "glyphosate". The American Heritage Dictionary of the English Language (5th ed.). HarperCollins. Retrieved June 28, 2020.
- ^ a b Glyphosate, Environmental Health Criteria monograph No. 159, Geneva: World Health Organization, 1994, ISBN 92-4-157159-4
- ^ Index no. 607-315-00-8 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at pp 570, 1100..
- ^ "2006-2007 Pesticide Market Estimates: Usage (Page 2) - Pesticides - US EPA". epa.gov. February 18, 2011. Archived from the original on June 26, 2015. Retrieved November 30, 2021.
- ^ Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vandenberg LN, vom Saal FS, Welshons WV, Benbroo CM (February 17, 2016). "Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement". Environmental Health. 15 (19): 13. Bibcode:2016EnvHe..15...19M. doi:10.1186/s12940-016-0117-0. PMC 4756530. PMID 26883814.
- ^ a b Cite error: The named reference
NatureonWHO2015was invoked but never defined (see the help page). - ^ "Report of the Joint Committee on Pesticide Residues, WHO/FAO, Geneva, 16 May, 2016" (PDF).
- ^ Tarazona, Jose V.; Court-Marques, Daniele; Tiramani, Manuela; Reich, Hermine; Pfeil, Rudolf; Istace, Frederique; Crivellente, Federica (April 3, 2017). "Glyphosate toxicity and carcinogenicity: a review of the scientific basis of the European Union assessment and its differences with IARC". Archives of Toxicology. 91 (8): 2723–43. doi:10.1007/s00204-017-1962-5. PMC 5515989. PMID 28374158.
- ^ Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K (May 2015). "Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate". The Lancet Oncology. 16 (5): 490–91. doi:10.1016/S1470-2045(15)70134-8. PMID 25801782.
- ^ Cite error: The named reference
IARC20March2015was invoked but never defined (see the help page). - ^ "Glyphosate" (PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 112. International Agency for Research on Cancer. August 11, 2016. Archived from the original (PDF) on July 30, 2019. Retrieved July 31, 2019.
- ^ "European Food Safety Authority – Glyphosate report" (PDF). EFSA. Retrieved May 23, 2016.
- ^ "Glyphosate: EFSA updates toxicological profile". European Food Safety Authority. November 12, 2015. Retrieved May 23, 2016.
- ^ "Glyphosate not classified as a carcinogen by ECHA". ECHA. March 15, 2017.