 Global Information
Global InformationEtanercept information
 | |
| Clinical data | |
|---|---|
| Trade names | Enbrel |
| Biosimilars | etanercept-szzs, etanercept-ykro, Benepali, Brenzys,[1] Erelzi,[2][3] Etacept, Etera,[4] Eticovo, Lifmior, Nepexto,[5] Rymti[6] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602013 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 58–76% (SC) |
| Elimination half-life | 70–132 hours |
| Identifiers | |
| CAS Number |
|
| PubChem SID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.224.383 |
| Chemical and physical data | |
| Formula | C2224H3475N621O698S36 |
| Molar mass | 51235.07 g·mol−1 |
Etanercept, sold under the brand name Enbrel among others, is a biologic medical product that is used to treat autoimmune diseases by interfering with tumor necrosis factor (TNF), a soluble inflammatory cytokine, by acting as a TNF inhibitor. It has US Food and Drug Administration (FDA) approval to treat rheumatoid arthritis, juvenile idiopathic arthritis and psoriatic arthritis, plaque psoriasis and ankylosing spondylitis. Tumor necrosis factor alpha (TNFα) is the "master regulator" of the inflammatory (immune) response in many organ systems. Autoimmune diseases are caused by an overactive immune response. Etanercept has the potential to treat these diseases by inhibiting TNF-alpha.[17]
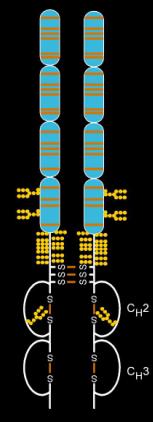
Etanercept is a fusion protein produced by recombinant DNA. It fuses the TNF receptor to the constant end of the IgG1 antibody. First, the developers isolated the DNA sequence that codes the human gene for soluble TNF receptor 2, which is a receptor that binds to tumor necrosis factor-alpha. Second, they isolated the DNA sequence that codes the human gene for the Fc end of immunoglobulin G1 (IgG1). Third, they linked the DNA for TNF receptor 2 to the DNA for IgG1 Fc. Finally, they expressed the linked DNA to produce a protein that links the protein for TNF receptor 2 to the protein for IgG1 Fc.[18]
The prototypic fusion protein was first synthesized and shown to be highly active and unusually stable as a modality for blockade of TNF in vivo in the early 1990s by Bruce A. Beutler, an academic researcher then at the University of Texas Southwestern Medical Center at Dallas, and his colleagues.[19][20]
These investigators also patented the protein,[21] selling all rights to its use to Immunex, a Seattle biotechnology company that was acquired by Amgen in 2002.[22]
It is a large molecule, with a molecular weight of 150 kDa, that binds to TNFα and decreases its role in disorders involving excess inflammation in humans and other animals, including autoimmune diseases such as ankylosing spondylitis,[23] juvenile rheumatoid arthritis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and, potentially, in a variety of other disorders mediated by excess TNFα. It is on the World Health Organization's List of Essential Medicines.[24]
- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Retrieved 7 April 2024.
- ^ "Arthritis". Health Canada. 8 May 2018. Retrieved 13 April 2024.
- ^ "Regulatory Decision Summary for Erelzi". Drug and Health Products Portal. 6 April 2017. Retrieved 13 April 2024.
- ^ a b Cite error: The named reference
Etera ARTGwas invoked but never defined (see the help page). - ^ a b "Nepexto EPAR". European Medicines Agency. 24 March 2020. Retrieved 4 March 2023.
- ^ a b Cite error: The named reference
Rymti ARTGwas invoked but never defined (see the help page). - ^ Cite error: The named reference
AusPAR: Etanerceptwas invoked but never defined (see the help page). - ^ "Etanercept Use During Pregnancy". Drugs.com. 24 January 2020. Retrieved 13 August 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Rymti Summary Basis of Decision". Health Canada. 23 October 2014. Retrieved 10 March 2023.
- ^ "Benepali 25 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC)". (emc). 25 January 2021. Retrieved 12 June 2021.
- ^ "Enbrel 25mg solution for injection in pre-filled pen - Summary of Product Characteristics (SmPC)". (emc). 8 June 2021. Retrieved 12 June 2021.
- ^ "Erelzi 50 mg solution for injection in pre filled pen - Summary of Product Characteristics (SmPC)". (emc). 25 May 2021. Retrieved 12 June 2021.
- ^ "Enbrel- etanercept solution Enbrel- etanercept kit". DailyMed. Retrieved 17 April 2021.
- ^ Cite error: The named reference
Enbrel EPARwas invoked but never defined (see the help page). - ^ "Nepexto Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ Feldmann M, Maini RN (October 2003). "Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases". Nature Medicine. 9 (10): 1245–1250. doi:10.1038/nm939. PMID 14520364. S2CID 52860838.
- ^ "Drugs@FDA: FDA-Approved Drugs".
- ^ Peppel K, Crawford D, Beutler B (December 1991). "A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity". The Journal of Experimental Medicine. 174 (6): 1483–1489. doi:10.1084/jem.174.6.1483. PMC 2119031. PMID 1660525.
- ^ Peppel K, Poltorak A, Melhado I, Jirik F, Beutler B (November 1993). "Expression of a TNF inhibitor in transgenic mice". Journal of Immunology. 151 (10): 5699–5703. doi:10.4049/jimmunol.151.10.5699. PMID 7693816. S2CID 10859938.
- ^ U.S. Patent number: 5,447,851
- ^ "Arthritis Drug Effective for Depression in Psoriasis Sufferers". Archived from the original on 2007-10-20. Retrieved 2008-01-10.
- ^ Braun J, McHugh N, Singh A, Wajdula JS, Sato R (June 2007). "Improvement in patient-reported outcomes for patients with ankylosing spondylitis treated with etanercept 50 mg once-weekly and 25 mg twice-weekly". Rheumatology. 46 (6): 999–1004. doi:10.1093/rheumatology/kem069. PMID 17389658.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.