 Global Information
Global InformationBohr model information
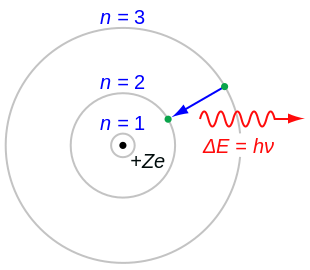
In atomic physics, the Bohr model or Rutherford–Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons. It is analogous to the structure of the Solar System, but with attraction provided by electrostatic force rather than gravity, and with the electron energies quantized (assuming only discrete values).
In the history of atomic physics, it followed, and ultimately replaced, several earlier models, including Joseph Larmor's Solar System model (1897), Jean Perrin's model (1901),[2] the cubical model (1902), Hantaro Nagaoka's Saturnian model (1904), the plum pudding model (1904), Arthur Haas's quantum model (1910), the Rutherford model (1911), and John William Nicholson's nuclear quantum model (1912). The improvement over the 1911 Rutherford model mainly concerned the new quantum mechanical interpretation introduced by Haas and Nicholson, but forsaking any attempt to explain radiation according to classical physics.
The model's key success lies in explaining the Rydberg formula for hydrogen's spectral emission lines. While the Rydberg formula had been known experimentally, it did not gain a theoretical basis until the Bohr model was introduced. Not only did the Bohr model explain the reasons for the structure of the Rydberg formula, it also provided a justification for the fundamental physical constants that make up the formula's empirical results.
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related quantum model was proposed by Arthur Erich Haas in 1910 but was rejected until the 1911 Solvay Congress where it was thoroughly discussed.[3] The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a mature quantum mechanics (1925) is often referred to as the old quantum theory.
- ^ Lakhtakia, Akhlesh; Salpeter, Edwin E. (1996). "Models and Modelers of Hydrogen". American Journal of Physics. 65 (9): 933. Bibcode:1997AmJPh..65..933L. doi:10.1119/1.18691.
- ^ Perrin, Jean (1901). "Les Hypothèses moléculaires". La Revue scientifique: 463.
- ^ de Broglie et al. 1912, pp. 122–123.