Global Information
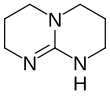
Global InformationTriazabicyclodecene information
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3,4,6,7,8-Hexahydro-2H-pyrimido[1,2-a]pyrimidine | |||
| Other names
1,5,7-Triazabicyclo[4.4.0]dec-5-ene
TBD Hexahydropyrimidopyrimidine hpp | |||
| Identifiers | |||
CAS Number
|
| ||
3D model (JSmol)
|
| ||
| ChEBI |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.024.880 | ||
| EC Number |
| ||
PubChem CID
|
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula
|
C7H13N3 | ||
| Molar mass | 139.20 g/mol | ||
| Melting point | 125 to 130 °C (257 to 266 °F; 398 to 403 K) | ||
| Acidity (pKa) | 15.2 ± 1.0[2] (pKa of conjugate acid in water); 26.03[3] (pKa of conjugate acid in acetonitrile) | ||
| Hazards | |||
| GHS labelling: | |||
Pictograms
|

| ||
Signal word
|
Danger | ||
Hazard statements
|
H314 | ||
Precautionary statements
|
P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |||
Triazabicyclodecene (1,5,7-triazabicyclo[4.4.0]dec-5-ene or TBD) is an organic compound consisting of a bicyclic guanidine. For a charge-neutral compound, it is a relatively strong base that is effective for a variety of organic transformations. TBD is colorless solid that is soluble in a variety of solvents.[4]
- ^ 1,5,7-Triazabicyclo[4.4.0]dec-5-ene at Sigma-Aldrich
- ^ Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta. 87 (4): 385–395. doi:10.5562/cca2472.
- ^ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- ^ Huczynski, Adam; Brzezinski, Bogumil (2008). "1,5,7-Triazabicyclo[4.4.0]dec-5-ene". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rn00786. ISBN 978-0-471-93623-7.