 Global Information
Global InformationRhodocene information
| |

| |
| Names | |
|---|---|
| IUPAC name
Rhodocene
| |
Other names
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C10H10Rh |
| Molar mass | 233.095 g·mol−1 |
| Appearance | yellow solid (dimer)[1] |
| Melting point | 174 °C (345 °F; 447 K) with decomposition (dimer)[1] |
Solubility in water
|
|
| Related compounds | |
Related compounds
|
ferrocene, cobaltocene, iridocene, bis(benzene)chromium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
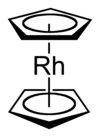
Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds.[2] The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.[1][3][4]
The history of organometallic chemistry includes the 19th-century discoveries of Zeise's salt[5][6][7] and nickel tetracarbonyl.[2] These compounds posed a challenge to chemists as the compounds did not fit with existing chemical bonding models. A further challenge arose with the discovery of ferrocene,[8] the iron analogue of rhodocene and the first of the class of compounds now known as metallocenes.[9] Ferrocene was found to be unusually chemically stable,[10] as were analogous chemical structures including rhodocenium, the unipositive cation of rhodocene[Note 1] and its cobalt and iridium counterparts.[11] The study of organometallic species including these ultimately led to the development of new bonding models that explained their formation and stability.[12][13] Work on sandwich compounds, including the rhodocenium-rhodocene system, earned Geoffrey Wilkinson and Ernst Otto Fischer the 1973 Nobel Prize for Chemistry.[14][15]
Owing to their stability and relative ease of preparation, rhodocenium salts are the usual starting material for preparing rhodocene and substituted rhodocenes, all of which are unstable. The original synthesis used a cyclopentadienyl anion and tris(acetylacetonato)rhodium(III);[11] numerous other approaches have since been reported, including gas-phase redox transmetalation[16] and using half-sandwich precursors.[17] Octaphenylrhodocene (a derivative with eight phenyl groups attached) was the first substituted rhodocene to be isolated at room temperature, though it decomposes rapidly in air. X-ray crystallography confirmed that octaphenylrhodocene has a sandwich structure with a staggered conformation.[18] Unlike cobaltocene, which has become a useful one-electron reducing agent in research,[19] no rhodocene derivative yet discovered is stable enough for such applications.
Biomedical researchers have examined the applications of rhodium compounds and their derivatives in medicine[20] and reported one potential application for a rhodocene derivative as a radiopharmaceutical to treat small cancers.[21][22] Rhodocene derivatives are used to synthesise linked metallocenes so that metal–metal interactions can be studied;[23] potential applications of these derivatives include molecular electronics and research into the mechanisms of catalysis.[24]
- ^ a b c d e f Cite error: The named reference
El_Murr_1979was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Crabtreewas invoked but never defined (see the help page). - ^ Cite error: The named reference
Fischer_1966was invoked but never defined (see the help page). - ^ Cite error: The named reference
Keller_1967was invoked but never defined (see the help page). - ^ Cite error: The named reference
Zeise discoverywas invoked but never defined (see the help page). - ^ Cite error: The named reference
Zeise reviewwas invoked but never defined (see the help page). - ^ Cite error: The named reference
DCW 1was invoked but never defined (see the help page). - ^ Cite error: The named reference
Hoffmanwas invoked but never defined (see the help page). - ^ Cite error: The named reference
FerroceneHistorywas invoked but never defined (see the help page). - ^ Cite error: The named reference
Pauson_Kealywas invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
JACS_1953was invoked but never defined (see the help page). - ^ Cite error: The named reference
Dewar-Chatt-Duncansonwas invoked but never defined (see the help page). - ^ Cite error: The named reference
ferrocene bondingwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Nobel Prizewas invoked but never defined (see the help page). - ^ Cite error: The named reference
New Scientist Nobel Prizewas invoked but never defined (see the help page). - ^ Cite error: The named reference
JACS_1982was invoked but never defined (see the help page). - ^ Cite error: The named reference
He_PhDwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Collins_1995was invoked but never defined (see the help page). - ^ Cite error: The named reference
cobaltoceniumwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Rh in medicinewas invoked but never defined (see the help page). - ^ Cite error: The named reference
Wenzelwas invoked but never defined (see the help page). - ^ Cite error: The named reference
organ distribwas invoked but never defined (see the help page). - ^ Cite error: The named reference
linked metalloceneswas invoked but never defined (see the help page). - ^ Cite error: The named reference
Wagner_2006was invoked but never defined (see the help page).
Cite error: There are <ref group=Note> tags on this page, but the references will not show without a {{reflist|group=Note}} template (see the help page).