 Global Information
Global InformationPlutonium information
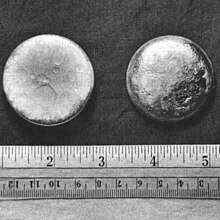 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Plutonium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /pluːˈtoʊniəm/ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allotropes | see Allotropes of plutonium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white, tarnishing to dark gray in air | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass number | [244] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Plutonium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | f-block groups (no number) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | f-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f6 7s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 24, 8, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 912.5 K (639.4 °C, 1182.9 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3505 K (3228 °C, 5842 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 19.85 g/cm3 (239Pu)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 16.63 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.82 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 333.5 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 35.5 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +2, +3, +4, +5, +6, +7, +8 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 159 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 187±1 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | monoclinic (mP16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constants |  b = 0.4822 nm c = 1.0964 nm β = 101.79° (at 20 °C)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 49.6×10−6/K (at 20 °C)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 6.74 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 1.460 µΩ⋅m (at 0 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 96 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 43 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2260 m/s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.21 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-07-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after dwarf planet Pluto, itself named after classical god of the underworld Pluto | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Glenn T. Seaborg, Arthur Wahl, Joseph W. Kennedy, Edwin McMillan (1940–1941) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of plutonium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen, silicon, and hydrogen. When exposed to moist air, it forms oxides and hydrides that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric. It is radioactive and can accumulate in bones, which makes the handling of plutonium dangerous.
Plutonium was first synthetically produced and isolated in late 1940 and early 1941, by a deuteron bombardment of uranium-238 in the 1.5-metre (60 in) cyclotron at the University of California, Berkeley. First, neptunium-238 (half-life 2.1 days) was synthesized, which subsequently beta-decayed to form the new element with atomic number 94 and atomic weight 238 (half-life 88 years). Since uranium had been named after the planet Uranus and neptunium after the planet Neptune, element 94 was named after Pluto, which at the time was considered to be a planet as well. Wartime secrecy prevented the University of California team from publishing its discovery until 1948.
Plutonium is the element with the highest atomic number known to occur in nature. Trace quantities arise in natural uranium-238 deposits when uranium-238 captures neutrons emitted by decay of other uranium-238 atoms. The heavy isotope plutonium-244 has a half-life long enough that extreme trace quantities should have survived primordially (from the Earth's formation) to the present, but so far experiments have not yet been sensitive enough to detect it.
Both plutonium-239 and plutonium-241 are fissile, meaning that they can sustain a nuclear chain reaction, leading to applications in nuclear weapons and nuclear reactors. Plutonium-240 exhibits a high rate of spontaneous fission, raising the neutron flux of any sample containing it. The presence of plutonium-240 limits a plutonium sample's usability for weapons or its quality as reactor fuel, and the percentage of plutonium-240 determines its grade (weapons-grade, fuel-grade, or reactor-grade). Plutonium-238 has a half-life of 87.7 years and emits alpha particles. It is a heat source in radioisotope thermoelectric generators, which are used to power some spacecraft. Plutonium isotopes are expensive and inconvenient to separate, so particular isotopes are usually manufactured in specialized reactors.
Producing plutonium in useful quantities for the first time was a major part of the Manhattan Project during World War II that developed the first atomic bombs. The Fat Man bombs used in the Trinity nuclear test in July 1945, and in the bombing of Nagasaki in August 1945, had plutonium cores. Human radiation experiments studying plutonium were conducted without informed consent, and several criticality accidents, some lethal, occurred after the war. Disposal of plutonium waste from nuclear power plants and dismantled nuclear weapons built during the Cold War is a nuclear-proliferation and environmental concern. Other sources of plutonium in the environment are fallout from numerous above-ground nuclear tests, which are now banned.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Magurno & Pearlstein 1981, pp. 835 ff.
