 Global Information
Global InformationHydroxymethylfurfural information
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
5-(Hydroxymethyl)furan-2-carbaldehyde[1] | |
| Other names
5-(Hydroxymethyl)-2-furaldehyde[1]
5-(Hydroxymethyl)furfural[1] | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
Beilstein Reference
|
110889 |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.000.595 |
| EC Number |
|
Gmelin Reference
|
278693 |
| KEGG |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C6H6O3 |
| Molar mass | 126.111 g·mol−1 |
| Appearance | Low melting white solid |
| Odor | Buttery, caramel |
| Density | 1.29 g/cm3 |
| Melting point | 30 to 34 °C (86 to 93 °F; 303 to 307 K) |
| Boiling point | 114 to 116 °C (237 to 241 °F; 387 to 389 K) (1 mbar) |
| UV-vis (λmax) | 284 nm[2] |
| Related compounds | |
Related furan-2-carbaldehydes
|
Furfural Methoxymethylfurfural |
| Hazards | |
| GHS labelling: | |
Pictograms
|
 [3] [3]
|
Signal word
|
Warning[3] |
Hazard statements
|
H315, H319, H335[3] |
Precautionary statements
|
P261, P305+P351+P338, P310[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
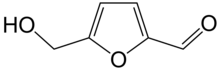
Hydroxymethylfurfural (HMF), also known as 5-(hydroxymethyl)furfural, is an organic compound formed by the dehydration of reducing sugars.[4][5] It is a white low-melting solid (although commercial samples are often yellow) which is highly soluble in both water and organic solvents. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups.
HMF can form in sugar-containing food, particularly as a result of heating or cooking. Its formation has been the topic of significant study as HMF was regarded as being potentially carcinogenic to humans. However, so far in vivo genotoxicity was negative. No relevance for humans concerning carcinogenic and genotoxic effects can be derived.[6] HMF is classified as a food improvement agent [7] and is primarily being used in the food industry in form of a food additive as a biomarker as well as a flavoring agent for food products.[8][9] It is also produced industrially on a modest scale[10] as a carbon-neutral feedstock for the production of fuels[11] and other chemicals.[12]
- ^ a b c "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 911. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ The Determination of HMF in Honey with an Evolution Array UV-Visible Spectrophotometer. Nicole Kreuziger Keppy and Michael W. Allen, Ph.D., Application note 51864, Thermo Fisher Scientific, Madison, WI, USA (article)
- ^ a b c d Sigma-Aldrich Co., 5-(Hydroxymethyl)furfural.
- ^ van Putten, Robert-Jan; van der Waal, Jan C.; de Jong, Ed; Rasrendra, Carolus B.; Heeres, Hero J.; de Vries, Johannes G. (2013). "Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources". Chemical Reviews. 113 (3): 1499–1597. doi:10.1021/cr300182k. ISSN 0009-2665. PMID 23394139.
- ^ Rosatella, Andreia A.; Simeonov, Svilen P.; Frade, Raquel F. M.; Afonso, Carlos A. M. (2011). "5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications". Green Chemistry. 13 (4): 754. doi:10.1039/c0gc00401d. ISSN 1463-9262.
- ^ Abraham, Klaus; Gürtler, Rainer; Berg, Katharina; Heinemeyer, Gerhard; Lampen, Alfonso; Appel, Klaus E. (2011-04-04). "Toxicology and risk assessment of 5-Hydroxymethylfurfural in food". Molecular Nutrition & Food Research. 55 (5): 667–678. doi:10.1002/mnfr.201000564. ISSN 1613-4125. PMID 21462333.
- ^ PubChem. "EU Food Improvement Agents - PubChem Data Source". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-06-25.
- ^ Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC Text with EEA relevance, 2012-10-02, retrieved 2018-06-25
- ^ Pubchem. "5-(Hydroxymethyl)-2-furaldehyde". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-06-25.
- ^ Kläusli, Thomas (2014). "AVA Biochem: commercialising renewable platform chemical 5-HMF". Green Processing and Synthesis. 3 (3): 235–236. doi:10.1515/gps-2014-0029. ISSN 2191-9550. S2CID 100848139.
- ^ Huber, George W.; Iborra, Sara; Corma, Avelino (2006). "Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering". Chem. Rev. 106 (9): 4044–98. doi:10.1021/cr068360d. PMID 16967928.MIT Technology Review
- ^ Lewkowski, J. (2001). "Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives". Arkivoc. 1: 17–54. doi:10.3998/ark.5550190.0002.102. hdl:2027/spo.5550190.0002.102. ISSN 1424-6376.