 Global Information
Global InformationGarenoxacin information
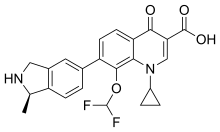 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
| Formula | C23H20F2N2O4 |
| Molar mass | 426.420 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
| | |
Garenoxacin (INN) is a quinolone antibiotic for the treatment of Gram-positive and Gram-negative bacterial infections.[1]
Garenoxacin was discovered by Toyama Chemical Co., Ltd. of Tokyo, Japan, and is currently being marketed in Japan under the tradename Geninax. Schering-Plough holds worldwide rights for garenoxacin, except for Japan, South Korea, and China.[citation needed]
On February 13, 2006, Schering-Plough announced that the United States Food and Drug Administration had accepted the New Drug Application (NDA) for garenoxacin, and had been granted a 10-month review.[2] As of 2015, however, it has not been approved in the US.[citation needed]
Schering-Plough later withdrew its application to the United States Food and Drug Administration, FDA, (August 20, 2006) for approval of the antibiotic Garenoxacin.[3]
The European Medicines Agency (EMA) had also been formally notified by Schering-Plough Europe (July 25, 2007) of its decision to withdraw the application for a centralized marketing authorization for garenoxacin as well.[4][5][6] Based on the CHMP review of the data regarding safety and efficacy (risk/benefit), the CHMP considered the application for garenoxacin to be unapprovable.[7]
- ^ Takagi H, Tanaka K, Tsuda H, Kobayashi H (December 2008). "Clinical studies of garenoxacin". International Journal of Antimicrobial Agents. 32 (6): 468–74. doi:10.1016/j.ijantimicag.2008.06.032. PMID 18790608.
- ^ "Drugs.com, Schering-Plough Reports Garenoxacin NDA Accepted for FDA Review". Retrieved 2008-03-25.
- ^ "Schering-Plough pulls its garenoxacin app".
- ^ "Schering-Plough Europe Withdraws Its Marketing Authorisation Application For Garenoxacin Mesylate". MediLexicon International Ltd. 28 July 2007. Archived from the original on 2007-08-08. Retrieved 2009-05-30.
- ^ "Garenoxacin mesylate: Withdrawn application". European Medicines Agency (EMA). 17 September 2018. Retrieved 13 July 2020.
- ^ "Schering-Plough Europe withdraws its marketing authorisation applicationfor Garenoxacin mesylate". European Medicines Agency (EMA) (Press release). Retrieved 13 July 2020.
- ^ "Withdrawal Assessment report for Garenoxacin Mesylate (garenoxacin)" (PDF). European Medicines Agency. 18 October 2007.