 Global Information
Global InformationCavitand information
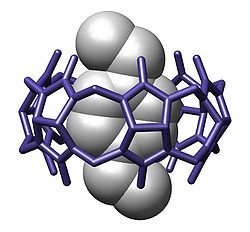
In chemistry, a cavitand is a container-shaped molecule.[2] The cavity of the cavitand allows it to engage in host–guest chemistry with guest molecules of a complementary shape and size. The original definition proposed by Cram includes many classes of molecules: cyclodextrins, calixarenes, pillararenes and cucurbiturils.[3] However, modern usage in the field of supramolecular chemistry specifically refers to cavitands formed on a resorcinarene scaffold by bridging adjacent phenolic units.[4] The simplest bridging unit is methylene (−CH2−), although dimethylene (−(CH2)2−), trimethylene (−(CH2)3−), benzal, xylyl, pyridyl, 2,3-disubstituted-quinoxaline, o-dinitrobenzyl, dialkylsilylene, and phosphonates are known. Cavitands that have an extended aromatic bridging unit, or an extended cavity containing 3 rows of aromatic rings are referred to as deep-cavity cavitands and have broad applications in host-guest chemistry.[5][6] These types of cavitands were extensively investigated by Rebek, and Gibb, among others.
- ^ Freeman, Wade A. (1984). "Structures of the p-xylylenediammonium chloride and calcium hydrogensulfate adducts of the cavitand 'cucurbituril', C36H36N24O12" (PDF). Acta Crystallographica Section B. 40 (4): 382–387. doi:10.1107/S0108768184002354.
- ^ D. J. Cram (1983). "Cavitands: organic hosts with enforced cavities". Science. 219 (4589): 1177–1183. Bibcode:1983Sci...219.1177C. doi:10.1126/science.219.4589.1177. PMID 17771285. S2CID 35255322.
- ^ Moran, John R.; Karbach, Stefan; Cram, Donald J. (October 1982). "Cavitands: synthetic molecular vessels". Journal of the American Chemical Society. 104 (21): 5826–5828. doi:10.1021/ja00385a064.
- ^ Jordan, J. H.; Gibb, B. C. (2017). "1.16 - Water-Soluble Cavitands☆". In Atwood, Jerry (ed.). Comprehensive Supramolecular Chemistry II. Elsevier. pp. 387–404. ISBN 9780128031995.
- ^ Wishard, A.; Gibb, B.C. (2016). "A chronology of cavitands". Calixarenes and beyond. Springer. pp. 195–234. doi:10.1007/978-3-319-31867-7_9. ISBN 978-3-319-31867-7.
- ^ Cai, X.; Gibb, B. C. (2017). "6.04 - Deep-Cavity Cavitands in Self-Assembly". In Atwood, Jerry (ed.). Comprehensive Supramolecular Chemistry II. Elsevier. pp. 75–82. ISBN 9780128031995.