 Global Information
Global InformationBotulinum toxin information
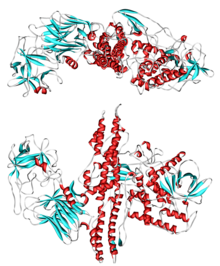 Ribbon diagram of tertiary structure of BotA (P0DPI1). PDB entry 3BTA. | |
| Clinical data | |
|---|---|
| Trade names | Botox, Myobloc, Jeuveau, others |
| Other names | BoNT, botox |
| Biosimilars | abobotulinumtoxinA, daxibotulinumtoxinA, daxibotulinumtoxinA-lanm, evabotulinumtoxinA, incobotulinumtoxinA, letibotulinumtoxinA, letibotulinumtoxinA-wlbg,[1] onabotulinumtoxinA, prabotulinumtoxinA, relabotulinumtoxinA, rimabotulinumtoxinB |
| AHFS/Drugs.com |
|
| MedlinePlus | a619021 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intramuscular, subcutaneous, intradermal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ECHA InfoCard | 100.088.372 |
| Chemical and physical data | |
| Formula | C6760H10447N1743O2010S32 |
| Molar mass | 149323.05 g·mol−1 |
| | |
| Bontoxilysin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.4.24.69 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Botulinum toxin, or botulinum neurotoxin (commonly called botox), is a highly potent neurotoxic protein produced by the bacterium Clostridium botulinum and related species.[23] It prevents the release of the neurotransmitter acetylcholine from axon endings at the neuromuscular junction, thus causing flaccid paralysis.[24] The toxin causes the disease botulism.[25] The toxin is also used commercially for medical and cosmetic purposes.[26][27] Botulinum toxin is an acetylcholine release inhibitor and a neuromuscular blocking agent.[1][22]
The seven main types of botulinum toxin are named types A to G (A, B, C1, C2, D, E, F and G).[26][28] New types are occasionally found.[29][30] Types A and B are capable of causing disease in humans, and are also used commercially and medically.[31][32][33] Types C–G are less common; types E and F can cause disease in humans, while the other types cause disease in other animals.[34]
Botulinum toxins are among the most potent toxins known to science.[35][36] Intoxication can occur naturally as a result of either wound or intestinal infection or by ingesting formed toxin in food. The estimated human median lethal dose of type A toxin is 1.3–2.1 ng/kg intravenously or intramuscularly, 10–13 ng/kg when inhaled, or 1000 ng/kg when taken by mouth.[37]
- ^ a b c "Letybo (letibotulinumtoxinA-wlbg) for injection, for intramuscular use" (PDF). Archived (PDF) from the original on 2 March 2024. Retrieved 2 March 2024.
- ^ a b Cite error: The named reference
Letybo APMDSwas invoked but never defined (see the help page). - ^ a b "Nuceiva". Therapeutic Goods Administration (TGA). 10 February 2023. Retrieved 8 April 2023.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Nuceiva (PPD Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 16 February 2023. Archived from the original on 18 March 2023. Retrieved 8 April 2023.
- ^ "Nuceiva prabotulinumtoxinA 100 Units Powder for Solution for Injection vial (381094)". Therapeutic Goods Administration (TGA). 26 January 2023. Archived from the original on 8 April 2023. Retrieved 8 April 2023.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 10 April 2023.
- ^ "Archived copy". Archived from the original on 31 March 2024. Retrieved 31 March 2024.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ "Regulatory Decision Summary - Botox". Health Canada. 23 October 2014. Archived from the original on 12 June 2022. Retrieved 12 June 2022.
- ^ "Regulatory Decision Summary - Nuceiva". Health Canada. 23 October 2014. Archived from the original on 7 June 2022. Retrieved 11 June 2022.
- ^ "Regulatory Decision Summary for Xeomin". Drug and Health Products Portal. 15 March 2022. Retrieved 1 April 2024.
- ^ "Regulatory Decision Summary for Botox". Drug and Health Products Portal. 7 February 2024. Archived from the original on 2 April 2024. Retrieved 2 April 2024.
- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Archived from the original on 7 April 2024. Retrieved 7 April 2024.
- ^ "Azzalure - Summary of Product Characteristics (SmPC)". (emc). 16 August 2022. Archived from the original on 18 December 2022. Retrieved 18 December 2022.
- ^ "Alluzience, 200 Speywood units/ml, solution for injection - Summary of Product Characteristics (SmPC)". (emc). 2 October 2022. Archived from the original on 18 December 2022. Retrieved 18 December 2022.
- ^ "Letybo 50 units powder for solution for injection - Summary of Product Characteristics (SmPC)". (emc). 10 May 2022. Archived from the original on 18 December 2022. Retrieved 18 December 2022.
- ^ "Xeomin 50 units powder for solution for injection - Summary of Product Characteristics (SmPC)". (emc). 28 July 2022. Archived from the original on 18 December 2022. Retrieved 18 December 2022.
- ^ "Botox- onabotulinumtoxina injection, powder, lyophilized, for solution". DailyMed. 30 July 2021. Archived from the original on 2 June 2022. Retrieved 12 June 2022.
- ^ "Botox Cosmetic- onabotulinumtoxina injection, powder, lyophilized, for solution". DailyMed. 9 February 2021. Archived from the original on 18 December 2022. Retrieved 18 December 2022.
- ^ "Myobloc- rimabotulinumtoxinb injection, solution". DailyMed. 22 March 2021. Archived from the original on 2 June 2022. Retrieved 12 June 2022.
- ^ "Dysport- botulinum toxin type a injection, powder, lyophilized, for solution". DailyMed. 28 February 2022. Archived from the original on 2 June 2022. Retrieved 12 June 2022.
- ^ a b "Daxxify- botulinum toxin type a injection, powder, lyophilized, for solution". DailyMed. 19 September 2022. Archived from the original on 28 September 2022. Retrieved 27 September 2022.
- ^ Montecucco C, Molgó J (June 2005). "Botulinal neurotoxins: revival of an old killer". Current Opinion in Pharmacology. 5 (3): 274–279. doi:10.1016/j.coph.2004.12.006. PMID 15907915.
- ^ Figgitt DP, Noble S (2002). "Botulinum toxin B: a review of its therapeutic potential in the management of cervical dystonia". Drugs. 62 (4): 705–722. doi:10.2165/00003495-200262040-00011. PMID 11893235. S2CID 46981635.
- ^ Cite error: The named reference
Shukla2004was invoked but never defined (see the help page). - ^ a b Janes LE, Connor LM, Moradi A, Alghoul M (April 2021). "Current Use of Cosmetic Toxins to Improve Facial Aesthetics". Plastic and Reconstructive Surgery. 147 (4): 644e–657e. doi:10.1097/PRS.0000000000007762. PMID 33776040. S2CID 232408799.
- ^ Cite error: The named reference
Al-Ghamdiwas invoked but never defined (see the help page). - ^ Rosales RL, Bigalke H, Dressler D (February 2006). "Pharmacology of botulinum toxin: differences between type A preparations". European Journal of Neurology. 13 (Suppl 1): 2–10. doi:10.1111/j.1468-1331.2006.01438.x. PMID 16417591. S2CID 32387953.
- ^ "Botulism toxin X: Time to update the textbooks, thanks to genomic sequencing". Boston Children's Hospital. 7 August 2017. Archived from the original on 14 September 2021. Retrieved 28 October 2019.
- ^ "Study: Novel botulinum toxin less dangerous than thought". CIDRAP. University of Minnesota. 17 June 2015. Archived from the original on 28 October 2019. Retrieved 28 October 2019.
- ^ Farag SM, Mohammed MO, El-Sobky TA, ElKadery NA, ElZohiery AK (March 2020). "Botulinum Toxin A Injection in Treatment of Upper Limb Spasticity in Children with Cerebral Palsy: A Systematic Review of Randomized Controlled Trials". JBJS Reviews. 8 (3): e0119. doi:10.2106/JBJS.RVW.19.00119. PMC 7161716. PMID 32224633.
- ^ Blumetti FC, Belloti JC, Tamaoki MJ, Pinto JA (October 2019). "Botulinum toxin type A in the treatment of lower limb spasticity in children with cerebral palsy". The Cochrane Database of Systematic Reviews. 2019 (10): CD001408. doi:10.1002/14651858.CD001408.pub2. PMC 6779591. PMID 31591703.
- ^ American Society of Health-System Pharmacists (27 October 2011). "OnabotulinumtoxinA (Botulinum Toxin Type A) Monograph for Professionals". drugs.com. Archived from the original on 6 September 2015. Retrieved 4 March 2015.
- ^ Cite error: The named reference
WHO2018was invoked but never defined (see the help page). - ^ Košenina S, Masuyer G, Zhang S, Dong M, Stenmark P (June 2019). "Crystal structure of the catalytic domain of the Weissella oryzae botulinum-like toxin". FEBS Letters. 593 (12): 1403–1410. doi:10.1002/1873-3468.13446. PMID 31111466.
- ^ Dhaked RK, Singh MK, Singh P, Gupta P (November 2010). "Botulinum toxin: Bioweapon & magic drug". Ncbi.NLM.nih.gov. 132 (5): 489–503. PMC 3028942. PMID 21149997.
- ^ Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, et al. (February 2001). "Botulinum toxin as a biological weapon: medical and public health management". JAMA. 285 (8): 1059–1070. doi:10.1001/jama.285.8.1059. PMID 11209178.