 Global Information
Global InformationPretomanid information
 | |
| Clinical data | |
|---|---|
| Trade names | Dovprela |
| Other names | PA-824 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619056 |
| License data |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
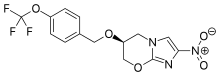
| Formula | C14H12F3N3O5 |
| Molar mass | 359.261 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
Pretomanid is an antibiotic medication used for the treatment of multi-drug-resistant tuberculosis affecting the lungs.[4][5] It is generally used together with bedaquiline and linezolid.[4] It is taken by mouth.[4]
The most common side effects include nerve damage, acne, vomiting, headache, low blood sugar, diarrhea, and liver inflammation.[4] It is in the nitroimidazole class of medications.[6]
Pretomanid was approved for medical use in the United States in August 2019,[4][7] and in the European Union in July 2020.[2] Pretomanid was developed by TB Alliance.[8][4][9] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[10] It is on the World Health Organization's List of Essential Medicines.[11]
- ^ Cite error: The named reference
Pretomanid FDA labelwas invoked but never defined (see the help page). - ^ a b "Pretomanid FGK EPAR". European Medicines Agency (EMA). 24 March 2020. Retrieved 25 September 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Dovprela Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ a b c d e f "FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs". U.S. Food and Drug Administration (FDA) (Press release). 14 August 2019. Retrieved 28 August 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Drug Trials Snapshots: Pretomanid". U.S. Food and Drug Administration (FDA). 14 August 2019. Retrieved 17 March 2020.
- ^ "Our Pipeline". TB Alliance. 19 July 2015. Retrieved 18 April 2019.
- ^ "Drug Approval Package: Pretomanid". U.S. Food and Drug Administration (FDA). 12 September 2019. Retrieved 25 September 2020.
- ^ "TB Medicine Pretomanid Enters Regulatory Review Process in the United States". TB Alliance. 8 March 2019. Retrieved 18 April 2019.
- ^ Abutaleb Y (14 August 2019). "New antibiotic approved for drug-resistant tuberculosis". The Washington Post.
- ^ "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration. 31 December 2019. Retrieved 15 September 2020.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.