 Global Information
Global InformationPMDTT information
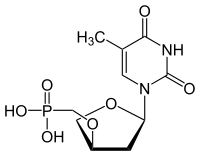
| |
| Names | |
|---|---|
| IUPAC name
({[(3R,5R)-5-(5-Methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)oxolan-3-yl]oxy}methyl)phosphonic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL |
|
| ChemSpider |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C10H15N2O7P |
| Molar mass | 306.211 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
PMDTT is an antiviral phosphonate nucleoside.[1]
- ^ Wu, Tongfei; Froeyen, Matheus; Kempeneers, Veerle; Pannecouque, Christophe; Wang, Jing; Busson, Roger; De Clercq, Erik; Herdewijn, Piet (13 April 2005). "Deoxythreosyl phosphonate nucleosides as selective anti-HIV agents". Journal of the American Chemical Society. 127 (14): 5056–5065. doi:10.1021/ja043045z. ISSN 0002-7863. PMID 15810840.