 Global Information
Global InformationOdevixibat information
 | |
| Clinical data | |
|---|---|
| Trade names | Bylvay |
| Other names | A4250 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a621049 |
| License data |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
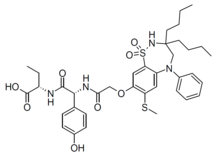
| Formula | C37H48N4O8S2 |
| Molar mass | 740.93 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
Odevixibat, sold under the brand name Bylvay, is a medication for the treatment of progressive familial intrahepatic cholestasis.[5][8] It is taken by mouth.[5] Odevixibat is a reversible, potent, selective inhibitor of the ileal bile acid transporter (IBAT).[8][9][10] It was developed by Albireo Pharma.[11]
The most common side effects include diarrhea, abdominal pain, hemorrhagic diarrhea, soft feces, and hepatomegaly (enlarged liver).[8]
Odevixibat was approved for medical use in the United States and in the European Union in July 2021.[5][6][7][12][13] The U.S. Food and Drug Administration considers it to be a first-in-class medication.[14]
- ^ "Details for: Bylvay". Health Canada. 30 October 2023. Retrieved 3 March 2024.
- ^ "Notice: Multiple additions to the Prescription Drug List (PDL) [2023-12-22]". Health Canada. 22 December 2023. Retrieved 3 January 2024.
- ^ "Regulatory Decision Summary for Bylvay". Drug and Health Products Portal. 23 October 2023. Retrieved 2 April 2024.
- ^ "Summary Basis of Decision for Bylvay". Drug and Health Products Portal. 1 September 2012. Retrieved 9 May 2024.
- ^ a b c d "Bylvay- odevixibat capsule, coated pellets". DailyMed. U.S. National Library of Medicine. Archived from the original on 29 July 2021. Retrieved 28 July 2021.
- ^ a b "Bylvay EPAR". European Medicines Agency (EMA). 20 April 2021. Archived from the original on 29 July 2021. Retrieved 28 July 2021.
- ^ a b "Bylvay". Union Register of medicinal products. Archived from the original on 24 July 2021. Retrieved 23 July 2021.
- ^ a b c "First treatment for rare liver disease". European Medicines Agency (EMA) (Press release). 21 May 2021. Retrieved 21 May 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Odevixibat". Albireo Pharma. Archived from the original on 22 May 2021. Retrieved 21 May 2021.
- ^ Karpen SJ, Kelly D, Mack C, Stein P (September 2020). "Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders". Hepatology International. 14 (5): 677–689. doi:10.1007/s12072-020-10070-w. PMID 32653991. S2CID 220481607.
- ^ Deeks ED (October 2021). "Odevixibat: First Approval". Drugs. 81 (15): 1781–1786. doi:10.1007/s40265-021-01594-y. PMC 8550539. PMID 34499340.
- ^ "Odevixibat: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 27 September 2021. Retrieved 23 July 2021.
- ^ "Albireo Announces FDA Approval of Bylvay (odevixibat), the First Drug Treatment for Patients With Progressive Familial Intrahepatic Cholestasis (PFIC)". Albireo Pharma (Press release). 20 July 2021. Retrieved 23 July 2021 – via GlobeNewswire.
- ^ Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (PDF). U.S. Food and Drug Administration (FDA) (Report). 13 May 2022. Archived from the original on 6 December 2022. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.