 Global Information
Global InformationGlucobrassicin information
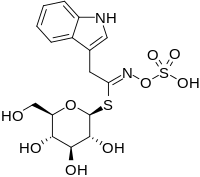
| |
| Names | |
|---|---|
| IUPAC name
1-S-[(1Z)-2-(1H-Indol-3-yl)-N-(sulfooxy)ethanimidoyl]-1-thio-β-D-glucopyranose
| |
| Other names
Indol-3-ylmethylglucosinolate
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.231.968 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C16H20N2O9S2 |
| Molar mass | 448.46 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Glucobrassicin is a type of glucosinolate that can be found in almost all cruciferous plants, such as cabbages, broccoli, mustards, and woad. As for other glucosinolates, degradation by the enzyme myrosinase is expected to produce an isothiocyanate, indol-3-ylmethylisothiocyanate. However, this specific isothiocyanate is expected to be highly unstable, and has indeed never been detected. The observed hydrolysis products when isolated glucobrassicin is degraded by myrosinase are indole-3-carbinol and thiocyanate ion (plus glucose, sulfate, and hydrogen ion), which are envisioned to result from a rapid reaction of the unstable isothiocyanate with water. However, a large number of other reaction products are known, and indole-3-carbinol is not the dominant degradation product when glucosinolate degradation takes place in crushed plant tissue[1] or in intact plants.[2][3]
Glucobrassicin is also known to be a highly active egg-laying stimulant of cabbage white butterflies such as the small white (Pieris rapae) and the large white (Pieris brassicae).
Several derivatives of glucobrassicin are known. The compound itself was first isolated from Brassica plants, hence the ending of the name. When a second, similar natural product was discovered, it was named neoglucobrassicin. When further derivatives were discovered, a more systematic nomenclature was used. Currently, the following six derivatives are known from plants:
- 1-Methoxyglucobrassicin (neoglucobrassicin)
- 4-Hydroxyglucobrassicin
- 4-Methoxyglucobrassicin
- 1,4-Dimethoxyglucobrassicin
- 1-Sulfoglucobrassicin
- 6′-Isoferuloylglucobrassicin
The three first mentioned derivatives are as frequent in crucifers as glucobrassicin itself. The additional three derivatives appear to be rare in nature. 4-methoxyglucobrassicin was recently reported to be a signal molecule involved in plant defence against bacteria and fungi.[2][3]
- ^ Agerbirk, Niels; Vos, Martin; Kim, Jae Hak; Jander, Georg (2008). "Indole glucosinolate breakdown and its biological effects". Phytochemistry Reviews. 8: 101. doi:10.1007/s11101-008-9098-0.
- ^ a b Clay, N. K.; Adio, A. M.; Denoux, C.; Jander, G.; Ausubel, F. M. (2009). "Glucosinolate Metabolites Required for an Arabidopsis Innate Immune Response". Science. 323 (5910): 95–101. Bibcode:2009Sci...323...95C. doi:10.1126/science.1164627. PMC 2630859. PMID 19095898.
- ^ a b Bednarek, P.; Pislewska-Bednarek, M.; Svatos, A.; Schneider, B.; Doubsky, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; Molina, A.; Schulze-Lefert, P. (2009). "A Glucosinolate Metabolism Pathway in Living Plant Cells Mediates Broad-Spectrum Antifungal Defense". Science. 323 (5910): 101–106. Bibcode:2009Sci...323..101B. doi:10.1126/science.1163732. PMID 19095900.