 Global Information
Global InformationFedratinib information
 | |
| Clinical data | |
|---|---|
| Trade names | Inrebic |
| Other names | SAR302503; TG101348 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Antineoplastic agent |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
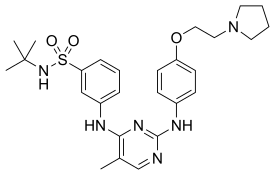
| Formula | C27H36N6O3S |
| Molar mass | 524.68 g·mol−1 |
| 3D model (JSmol) |
|
| Density | 1.247 ± 0.06 g/cm3 |
SMILES
| |
InChI
| |
| | |
Fedratinib, sold under the brand name Inrebic, is an anti-cancer medication used to treat myeloproliferative diseases including myelofibrosis.[5] It is used in the form of fedratinib hydrochloride capsules that are taken by mouth. It is a semi-selective inhibitor of Janus kinase 2 (JAK-2).[5][6] It was approved by the FDA on 16 August 2019.[5]
Myelofibrosis is a myeloid cancer associated with anemia, splenomegaly, and constitutional symptoms. Patients with myelofibrosis frequently harbor mutations which activate the JAK-STAT signaling pathway and which are sensitive to fedratinib. Phase I trial results focused on safety and efficacy of fedratinib in patients with high- or intermediate-risk primary or post–polycythemia vera/essential thrombocythemia myelofibrosis have been published in 2011.[7]
- ^ "Summary Basis of Decision (SBD) for Inrebic". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Inrebic- fedratinib hydrochloride capsule". DailyMed. Retrieved 3 March 2021.
- ^ "Inrebic EPAR". European Medicines Agency (EMA). 9 December 2020. Retrieved 3 March 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Inrebic Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ a b c "FDA approves treatment for patients with rare bone marrow disorder". U.S. Food and Drug Administration (FDA) (Press release). 16 August 2019. Archived from the original on 21 November 2019. Retrieved 16 August 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Drug Trials Snapshots: Inrebic". U.S. Food and Drug Administration (FDA). 30 August 2019. Archived from the original on 21 November 2019. Retrieved 20 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, Silverman MH, Gilliland DG, Shorr J, Tefferi A (March 2011). "Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis". Journal of Clinical Oncology. 29 (7): 789–796. doi:10.1200/JCO.2010.32.8021. PMC 4979099. PMID 21220608.