 Global Information
Global InformationDiethylstilbestrol information
 | |
 | |
| Clinical data | |
|---|---|
| Other names | DES; Stilboestrol; Stilbestrol; (E)-11,12-Diethyl-4,13-stilbenediol |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth, vaginal, topical, intravenous, intramuscular injection (as an ester) |
| Drug class | Nonsteroidal estrogen |
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed[1] |
| Protein binding | >95%[2] |
| Metabolism | Hydroxylation, oxidation, glucuronidation[1][2][3] |
| Metabolites | • (Z,Z)-Dienestrol[1] • Paroxypropione[1] • Glucuronides[2][3] |
| Elimination half-life | 24 hours[1][4] |
| Excretion | Urine, feces[2][3] |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.000.253 |
| Chemical and physical data | |
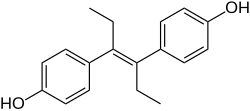
| Formula | C18H20O2 |
| Molar mass | 268.356 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
| (verify) | |
Diethylstilbestrol (DES), also known as stilbestrol or stilboestrol, is a nonsteroidal estrogen medication, which is presently rarely used.[5][6][7] In the past, it was widely used for a variety of indications, including pregnancy support for those with a history of recurrent miscarriage, hormone therapy for menopausal symptoms and estrogen deficiency, treatment of prostate cancer and breast cancer, and other uses.[5] By 2007, it was only used in the treatment of prostate cancer and breast cancer.[8] In 2011, Hoover and colleagues reported on adverse health outcomes linked to DES including infertility, miscarriage, ectopic pregnancy, preeclampsia, preterm birth, stillbirth, infant death, menopause prior to age 45, breast cancer, cervical cancer, and vaginal cancer.[9] While most commonly taken by mouth, DES was available for use by other routes as well, for instance, vaginal, topical, and by injection.
DES is an estrogen, or an agonist of the estrogen receptors, the biological target of estrogens like estradiol.[7] It is a synthetic and nonsteroidal estrogen of the stilbestrol group, and differs from the natural estrogen estradiol in various ways.[7] Compared to estradiol, DES has greatly improved bioavailability when taken by mouth, is more resistant to metabolism, and shows relatively increased effects in certain parts of the body like the liver and uterus.[7] These differences result in DES having an increased risk of blood clots, cardiovascular issues, and certain other adverse effects.[7]
DES was discovered in 1938 and introduced for medical use in 1939.[10][11] From about 1940 to 1971, the medication was given to pregnant women in the incorrect belief that it would reduce the risk of pregnancy complications and losses.[10] In 1971, DES was shown to cause clear-cell carcinoma, a rare vaginal tumor, in those who had been exposed to this medication in utero.[10][5] The United States Food and Drug Administration subsequently withdrew approval of DES as a treatment for pregnant women.[10][5] Follow-up studies have indicated that DES also has the potential to cause a variety of significant adverse medical complications during the lifetimes of those exposed, including XY genital undermasculinization.[10][12]
The United States National Cancer Institute recommends[13] children born to mothers who took DES to undergo special medical exams on a regular basis to screen for complications as a result of the medication. Individuals who were exposed to DES during their mothers' pregnancies are commonly referred to as "DES daughters" and "DES sons".[10][14] Since the discovery of the toxic effects of DES, it has largely been discontinued and is now mostly no longer marketed.[10][15]
- ^ a b c d e Cite error: The named reference
ChabnerLongo1996was invoked but never defined (see the help page). - ^ a b c d Cite error: The named reference
OelschlägerRothley1988was invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
pmid8428334was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid7154205was invoked but never defined (see the help page). - ^ a b c d Cite error: The named reference
pmid4276416was invoked but never defined (see the help page). - ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 396–. ISBN 978-1-4757-2085-3.
- ^ a b c d e Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324. Archived (PDF) from the original on 2016-08-22. Retrieved 2018-02-24.
- ^ Watkins ES (16 April 2007). The Estrogen Elixir: A History of Hormone Replacement Therapy in America. JHU Press. pp. 26–. ISBN 978-0-8018-8602-7. Archived from the original on 12 May 2024. Retrieved 3 September 2020.
- ^ "Effects of Diethylstilbestrol (DES), a Trans-placental Carcinogen". dceg.cancer.gov. 20 November 2012. Archived from the original on 8 February 2023. Retrieved 3 September 2020.
- ^ a b c d e f g Veurink M, Koster M, Berg LT (June 2005). "The history of DES, lessons to be learned". Pharm World Sci. 27 (3): 139–43. doi:10.1007/s11096-005-3663-z. PMID 16096877. S2CID 12630813.
- ^ Feldberg GD, Ladd-Taylor M, Li A (2003). Women, Health and Nation: Canada and the United States Since 1945. McGill-Queen's Press - MQUP. pp. 103–. ISBN 978-0-7735-2501-6. Archived from the original on 2023-01-14. Retrieved 2020-09-03.
- ^ "DES Update: For Consumers". United States Department of Health and Human Services: Centers for Disease Control and Prevention. Archived from the original on 2020-12-11. Retrieved 2011-06-30.
- ^ "Diethylstilbestrol (DES) and Cancer". National Cancer Institute. Archived from the original on 2015-02-23. Retrieved 2011-06-30.
- ^ Arnold A (January 5, 2017). "The Devastating Effects of a 1940s 'Wonder Pill' Haunt Women Generations Later". Broadly. Archived from the original on August 14, 2020. Retrieved July 27, 2019.
- ^ Coelingh Bennink HJ (April 2004). "Are all estrogens the same?". Maturitas. 47 (4): 269–275. doi:10.1016/j.maturitas.2003.11.009. PMID 15063479.