 Global Information
Global InformationChugaev elimination information
| Chugaev elimination | |
|---|---|
| Named after | Lev Chugaev |
| Reaction type | Elimination reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000538 |
The Chugaev elimination is a chemical reaction that involves the elimination of water from alcohols to produce alkenes. The intermediate is a xanthate. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev (1873–1922), who first reported the reaction sequence in 1899.

In the first step, a xanthate salt is formed out of the alkoxide and carbon disulfide (CS2). With the addition of iodomethane, the alkoxide is transformed into a methyl xanthate.

At about 200 °C, the alkene is formed by an intramolecular elimination. In a 6-membered cyclic transition state the hydrogen atom is removed from the carbon atom β to the xanthate oxygen in a syn-elimination. The side product decomposes to carbonyl sulfide (OCS) and methanethiol.
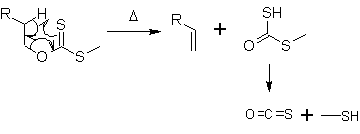
The Chugaev elimination is similar in mechanism to other thermal elimination reactions such as the Cope elimination and ester pyrolysis. Xanthates typically undergo elimination from 120 to 200 °C, while esters typically require 400 to 500 °C and amine oxides routinely react between 80 and 160 °C.
In the development of Cram's rule, structural assignment of the reaction products were made by applying Chugaev elimination, wherein the threo isomer reacts to the cis isomer of -α-methyl-stilbene and the erythro isomer to the trans version.[1]
- ^ Cram, Donald J.; Elhafez, Fathy Ahmed Abd (1952). "Studies in Stereochemistry. X. The Rule of "Steric Control of Asymmetric Induction" in the Syntheses of Acyclic Systems". Journal of the American Chemical Society. 74 (23): 5828–5835. doi:10.1021/ja01143a007.
