 Global Information
Global InformationBorepin information
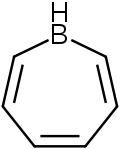
| |
| Names | |
|---|---|
| IUPAC name
1H-borepine
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C6H7B |
| Molar mass | 89.93 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
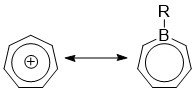
Borepins are a class of boron-containing heterocycles used in main group chemistry. They consist of a seven-membered unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene, which is a seven-membered ring containing three carbon-carbon double bonds, each of which contributes 2π electrons for a total of 6π electrons. Unlike other seven-membered systems such as silepins and phosphepins, boron has a vacant p-orbital that can interact with the π and π* orbitals of the cycloheptatriene.[1][2][3] This leads to an isoelectronic state akin to that of the tropylium cation, aromatizing the borepin while also allowing it to act as a Lewis acid.[3] The aromaticity of borepin is relatively weak compared to traditional aromatics such as benzene or even cycloheptatriene, which has led to the synthesis of many fused, π-conjugated borepin systems over the years.[2][3][4][5][6][7] Simple and complex borepins have been extensively studied more recently due to their high fluorescence and potential applications in technologies like organic light-emitting diodes (OLEDs) and photovoltaic cells.[3][5][7][8][9]
- ^ Schulman, Jerome M.; Disch, Raymond L. (March 1989). "Thermochemistry of borabenzene and borepin". Organometallics. 8 (3): 733–737. doi:10.1021/om00105a024.
- ^ a b Ashe, A. J.; Drone, F. J.; Kausch, C. M.; Kroker, J.; Al-Taweel, S. M. (1 January 1990). "Borepins and group 15 element heteroles". Pure and Applied Chemistry. 62 (3): 513–517. doi:10.1351/pac199062030513. S2CID 96223530.
- ^ a b c d Wang, Lili; Ma, Juan; Si, Erbing; Duan, Zheng (February 2021). "Recent Advances in Luminescent Annulated Borepins, Silepins, and Phosphepins". Synthesis. 53 (4): 623–635. doi:10.1055/s-0040-1705946. S2CID 228982156.
- ^ Schickedanz, Kai; Radtke, Julian; Bolte, Michael; Lerner, Hans-Wolfram; Wagner, Matthias (22 February 2017). "Facile Route to Quadruply Annulated Borepins". Journal of the American Chemical Society. 139 (7): 2842–2851. doi:10.1021/jacs.7b00268. PMID 28125773.
- ^ a b Mercier, Lauren G.; Piers, Warren E.; Parvez, Masood (29 July 2009). "Benzo- and Napthoborepins: Blue-Emitting Boron Analogues of Higher Acenes". Angewandte Chemie International Edition. 48 (33): 6108–6111. doi:10.1002/anie.200902803. PMID 19598197.
- ^ Eisch, John J.; Galle, James E. (July 1975). "Rearrangements of organometallic compounds. XIII. Boraaromatic systems. IV. Synthesis of heptaphenylborepin via the thermal rearrangement of heptaphenyl-7-borabicyclo[2.2.1]heptadiene". Journal of the American Chemical Society. 97 (15): 4436–4437. doi:10.1021/ja00848a070.
- ^ a b Adachi, Yohei; Ohshita, Joji (26 March 2018). "Synthesis and Properties of Benzo[ d ]dithieno[ b , f ]borepins". Organometallics. 37 (6): 869–881. doi:10.1021/acs.organomet.7b00844.
- ^ Yang, Wenlong; Krantz, Kelsie E.; Freeman, Lucas A.; Dickie, Diane A.; Molino, Andrew; Kaur, Aishvaryadeep; Wilson, David J. D.; Gilliard, Robert J. (25 September 2019). "Stable Borepinium and Borafluorenium Heterocycles: A Reversible Thermochromic "Switch" Based on Boron–Oxygen Interactions". Chemistry – A European Journal. 25 (54): 12512–12516. doi:10.1002/chem.201903348. PMID 31334883. S2CID 198170504.
- ^ Li, Chenglong; Shi, Yafei; Li, Pengfei; Zhang, Niu; Wang, Nan; Yin, Xiaodong; Chen, Pangkuan (17 September 2021). "Access to Highly Luminescent N-Doped Diazaborepins with Penta-, Hexa-, and Heptagon Substructures". Organic Letters. 23 (18): 7123–7128. doi:10.1021/acs.orglett.1c02528. PMID 34449226. S2CID 237339643.