 Global Information
Global InformationBicalutamide information
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | Bicalutamide: • /ˌbaɪkəˈluːtəmaɪd/[1] • BY-kə-LOO-tə-myde[1] |
| Trade names | Casodex, Calutex, others |
| Other names | ICI-176,334; ZD-176,334 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697047 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth[2] |
| Drug class | Nonsteroidal antiandrogen |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed; absolute bioavailability unknown[3] |
| Protein binding | Racemate: 96.1%[2] (R)-Isomer: 99.6%[2] (Mainly to albumin)[2] |
| Metabolism | Liver (extensively):[4][9] • Hydroxylation (CYP3A4) • Glucuronidation (UGT1A9) |
| Metabolites | • Bicalutamide glucuronide • Hydroxybicalutamide • Hydroxybicalutamide gluc. (All inactive)[4][2][5][6] |
| Elimination half-life | Single-dose: 5.8 days[7] Continuous: 7–10 days[8] |
| Excretion | Feces: 43%[4] Urine: 34%[4] |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| PDB ligand |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.126.100 |
| Chemical and physical data | |
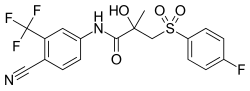
| Formula | C18H14F4N2O4S |
| Molar mass | 430.37 g·mol−1 |
| 3D model (JSmol) |
|
| Chirality | Racemic mixture (of (R)- and (S)-enantiomers) |
| Melting point | 191 to 193 °C (376 to 379 °F) (experimental) |
| Boiling point | 650 °C (1,202 °F) (predicted) |
| Solubility in water | 0.005 |
SMILES
| |
InChI
| |
| (verify) | |
Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer.[10] It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat metastatic prostate cancer (mPC).[11][10][12] To a lesser extent, it is used at high doses for locally advanced prostate cancer (LAPC) as a monotherapy without castration.[4][2][13] Bicalutamide was also previously used as monotherapy to treat localized prostate cancer (LPC), but authorization for this use was withdrawn following unfavorable trial findings.[13][14][15][16] Besides prostate cancer, bicalutamide is limitedly used in the treatment of excessive hair growth and scalp hair loss in women,[17][18] as a puberty blocker and component of feminizing hormone therapy for transgender girls and women,[19] to treat gonadotropin-independent early puberty in boys,[20] and to prevent overly long-lasting erections in men.[21] It is taken by mouth.[10]
Common side effects of bicalutamide in men include breast growth, breast tenderness, and hot flashes.[10] Other side effects in men include feminization and sexual dysfunction.[22][23] Some side effects like breast changes and feminization are minimal when combined with castration.[24] While the medication appears to produce few side effects in women, its use in women is not explicitly approved by the Food and Drug Administration (FDA) at this time.[25][10] Use during pregnancy may harm the baby.[10] In men with early prostate cancer, bicalutamide monotherapy has been found to increase the likelihood of death from causes other than prostate cancer.[26][13] Bicalutamide produces abnormal liver changes necessitating discontinuation in around 1% of people.[27][13] Rarely, it has been associated with cases of serious liver damage,[10] serious lung toxicity,[3] and sensitivity to light.[28][29] Although the risk of adverse liver changes is small, monitoring of liver function is recommended during treatment.[10]
Bicalutamide is a member of the nonsteroidal antiandrogen (NSAA) group of medications.[3] It works by selectively blocking the androgen receptor (AR), the biological target of the androgen sex hormones testosterone and dihydrotestosterone (DHT).[30] It does not lower androgen levels.[3] The medication can have some estrogen-like effects in men when used as a monotherapy due to increased estradiol levels.[31][32][33] Bicalutamide is well-absorbed, and its absorption is not affected by food.[2] The elimination half-life of the medication is around one week.[2][10] It shows peripheral selectivity in animals, but crosses the blood–brain barrier and affects both the body and brain in humans.[2][34]
Bicalutamide was patented in 1982 and approved for medical use in 1995.[35] It is on the World Health Organization's List of Essential Medicines.[36] Bicalutamide is available as a generic medication.[37] The drug is sold in more than 80 countries, including most developed countries.[38][39][40] It was at one time the most widely used antiandrogen in the treatment of prostate cancer, with millions of men with the disease having been prescribed it.[23][41][42][43][44] Although bicalutamide is also used for other indications besides prostate cancer, the vast majority of prescriptions appear to be for treatment of prostate cancer.[44]
- ^ a b Finkel R, Clark MA, Cubeddu LX (2009). Pharmacology. Lippincott Williams & Wilkins. pp. 481–. ISBN 978-0-7817-7155-9.
- ^ a b c d e f g h i Cockshott ID (2004). "Bicalutamide: clinical pharmacokinetics and metabolism". Clinical Pharmacokinetics. 43 (13): 855–878. doi:10.2165/00003088-200443130-00003. PMID 15509184. S2CID 29912565.
- ^ a b c d Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 497, 521. ISBN 978-0-7817-2845-4. Archived from the original on 11 May 2016.
- ^ a b c d e Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 121, 1288, 1290. ISBN 978-0-7817-6879-5. Archived from the original on 8 September 2017.
- ^ Dole EJ, Holdsworth MT (1997). "Nilutamide: an antiandrogen for the treatment of prostate cancer". The Annals of Pharmacotherapy. 31 (1): 65–75. doi:10.1177/106002809703100112. PMID 8997470. S2CID 20347526.
page 67: Currently, information is not available regarding the activity of the major urinary metabolites of bicalutamide, bicalutamide glucuronide, and hydroxybicalutamide glucuronide.
- ^ Schellhammer PF (September 2002). "An evaluation of bicalutamide in the treatment of prostate cancer". Expert Opinion on Pharmacotherapy. 3 (9): 1313–28. doi:10.1517/14656566.3.9.1313. PMID 12186624. S2CID 32216411.
The clearance of bicalutamide occurs pre- dominantly by hepatic metabolism and glucuronidation, with excretion of the resulting inactive metabolites in the urine and faces.
- ^ Cite error: The named reference
Skidmore-Roth2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
JordanFurr2010was invoked but never defined (see the help page). - ^ Cite error: The named reference
GrosseCampeau2013was invoked but never defined (see the help page). - ^ a b c d e f g h i "Bicalutamide". The American Society of Health-System Pharmacists. Archived from the original on 29 December 2016. Retrieved 8 December 2016.
- ^ Wass JA, Stewart PM (28 July 2011). Oxford Textbook of Endocrinology and Diabetes. OUP Oxford. pp. 1625–. ISBN 978-0-19-923529-2. Archived from the original on 11 May 2016.
- ^ Shergill I, Arya M, Grange PR, Mundy AR (2010). Medical Therapy in Urology. Springer Science & Business Media. p. 40. ISBN 9781848827042. Archived from the original on 28 October 2014.
- ^ a b c d Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer" (PDF). Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. S2CID 46966712. Archived from the original (PDF) on 28 August 2016. Retrieved 13 August 2016.
- ^ Cite error: The named reference
pmid18093474was invoked but never defined (see the help page). - ^ Cite error: The named reference
BowsherCarter2008was invoked but never defined (see the help page). - ^ Cite error: The named reference
NargundRaghavan2015was invoked but never defined (see the help page). - ^ Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B (22 January 2009). Evidence-Based Dermatology. John Wiley & Sons. pp. 529–. ISBN 978-1-4443-0017-8. Archived from the original on 2 May 2016.
- ^ Carvalho RM, Santos LD, Ramos PM, Machado CJ, Acioly P, Frattini SC, Barcaui CB, Donda AL, Melo DF (January 2022). "Bicalutamide and the new perspectives for female pattern hair loss treatment: What dermatologists should know". J Cosmet Dermatol. 21 (10): 4171–4175. doi:10.1111/jocd.14773. PMID 35032336. S2CID 253239337.
- ^ Randolph JF (December 2018). "Gender-Affirming Hormone Therapy for Transgender Females". Clin Obstet Gynecol. 61 (4): 705–721. doi:10.1097/GRF.0000000000000396. PMID 30256230. S2CID 52821192.
- ^ Jameson JL, De Groot LJ (25 February 2015). Edndocrinology: Adult and Pediatric. Elsevier Health Sciences. pp. 2425–2426, 2139. ISBN 978-0-323-32195-2.
- ^ Yuan J, Desouza R, Westney OL, Wang R (2008). "Insights of priapism mechanism and rationale treatment for recurrent priapism". Asian Journal of Andrology. 10 (1): 88–101. doi:10.1111/j.1745-7262.2008.00314.x. PMID 18087648.
- ^ Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW (2010). "Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life". The Journal of Sexual Medicine. 7 (9): 2996–3010. doi:10.1111/j.1743-6109.2010.01902.x. PMID 20626600.
- ^ a b Hammerer P, Manka L (2019). "Androgen Deprivation Therapy for Advanced Prostate Cancer". Urologic Oncology. Springer International Publishing. pp. 255–276. doi:10.1007/978-3-319-42623-5_77. ISBN 978-3-319-42622-8.
Bicalutamide is the most widely used antiandrogen in the treatment of prostate cancer. [...] Common side effects [of bicalutamide] include breast enlargement, breast tenderness, hot flashes, and constipation as well as feminization and changes in mood and liver as well as lung toxicity; monitoring of liver enzymes is recommended during treatment (Schellhammer and Davis 2004).
- ^ Droz J, Audisio RA (2 October 2012). Management of Urological Cancers in Older People. Springer Science & Business Media. pp. 84–. ISBN 978-0-85729-986-4. Archived from the original on 11 May 2016.
- ^ Shapiro J (12 November 2012). Hair Disorders: Current Concepts in Pathophysiology, Diagnosis and Management, An Issue of Dermatologic Clinics. Elsevier Health Sciences. pp. 187–. ISBN 978-1-4557-7169-1.
- ^ Cite error: The named reference
pmid35569476was invoked but never defined (see the help page). - ^ "Casodex- bicalutamide tablet". DailyMed. 1 September 2019. Archived from the original on 27 July 2020. Retrieved 7 May 2020.
- ^ Lee K, Oda Y, Sakaguchi M, Yamamoto A, Nishigori C (May 2016). "Drug-induced photosensitivity to bicalutamide – case report and review of the literature". Photodermatology, Photoimmunology & Photomedicine. 32 (3): 161–4. doi:10.1111/phpp.12230. PMID 26663090. S2CID 2761388.
- ^ Lee K, et al. (2016). "Drug-induced photosensitivity to bicalutamide – case report and review of the literature". Reactions Weekly. 1612 (1): 161–4. doi:10.1007/s40278-016-19790-1. PMID 26663090. S2CID 261402820.
- ^ Singh SM, Gauthier S, Labrie F (February 2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Current Medicinal Chemistry. 7 (2): 211–47. doi:10.2174/0929867003375371. PMID 10637363.
- ^ Strauss III JF, Barbieri RL (28 August 2013). Yen & Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 688–. ISBN 978-1-4557-5972-9. Archived from the original on 14 January 2023. Retrieved 27 September 2016.
Bone density improves in men receiving bicalutamide, most likely secondary to the 146% increase in estradiol and the fact that estradiol is the major mediator of bone density in men.
- ^ Cite error: The named reference
MarcusFeldman2007was invoked but never defined (see the help page). - ^ Cite error: The named reference
MahlerVerhelst1998was invoked but never defined (see the help page). - ^ Cite error: The named reference
FurrTucker1996was invoked but never defined (see the help page). - ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 515. ISBN 9783527607495. Archived from the original on 12 January 2023. Retrieved 24 August 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 381. ISBN 9781284057560.
- ^ "Bicalutamide – International Drug Names". Drugs.com. Archived from the original on 18 September 2016. Retrieved 13 August 2016.
- ^ Akaza H (1999). "[A new anti-androgen, bicalutamide (Casodex), for the treatment of prostate cancer—basic clinical aspects]". Gan to Kagaku Ryoho. Cancer & Chemotherapy (in Japanese). 26 (8): 1201–7. PMID 10431591.
- ^ "1999 Annual Report and Form 20-F" (PDF). AstraZeneca. Archived (PDF) from the original on 9 September 2017. Retrieved 1 July 2017.
- ^ Mukherji D, Pezaro CJ, De-Bono JS (February 2012). "MDV3100 for the treatment of prostate cancer". Expert Opinion on Investigational Drugs. 21 (2): 227–33. doi:10.1517/13543784.2012.651125. PMID 22229405. S2CID 46339544.
- ^ Pchejetski D, Alshaker H, Stebbing J (2014). "Castrate-resistant prostate cancer: the future of antiandrogens" (PDF). Trends in Urology & Men's Health. 5 (1): 7–10. doi:10.1002/tre.371. S2CID 57988002. Archived (PDF) from the original on 19 July 2018. Retrieved 11 December 2019.
- ^ Campbell T (22 January 2014). "Slowing Sales for Johnson & Johnson's Zytiga May Be Good News for Medivation". The Motley Fool. Archived from the original on 26 August 2016. Retrieved 20 July 2016.
[...] the most commonly prescribed treatment for metastatic castration resistant prostate cancer: bicalutamide. That was sold as AstraZeneca's billion-dollar-a-year drug Casodex before losing patent protection in 2008. AstraZeneca still generates a few hundred million dollars in sales from Casodex, [...]
- ^ a b Chang S (10 March 2010), Bicalutamide BPCA Drug Use Review in the Pediatric Population (PDF), U.S. Department of Health and Human Service, archived (PDF) from the original on 24 October 2016, retrieved 20 July 2016